Immune Netw.
2009 Dec;9(6):274-284. 10.4110/in.2009.9.6.274.
Swiprosin-1 Regulates Cytokine Expression of Human Mast Cell Line HMC-1 through Actin Remodeling
- Affiliations
-
- 1Department of Life Science, Gwangju Institute of Science and Technology, Gwangju 500-712, Korea.
- 2Department of Internal Medicine, Chonbuk National University College of Medicine, Jeonju 561-712, Korea. clickm@jbnu.ac.kr
- KMID: 2150656
- DOI: http://doi.org/10.4110/in.2009.9.6.274
Abstract
- BACKGROUND
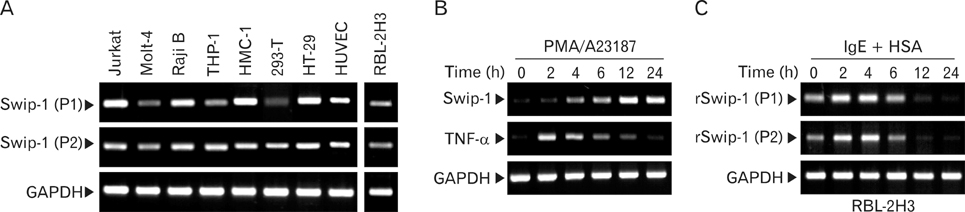
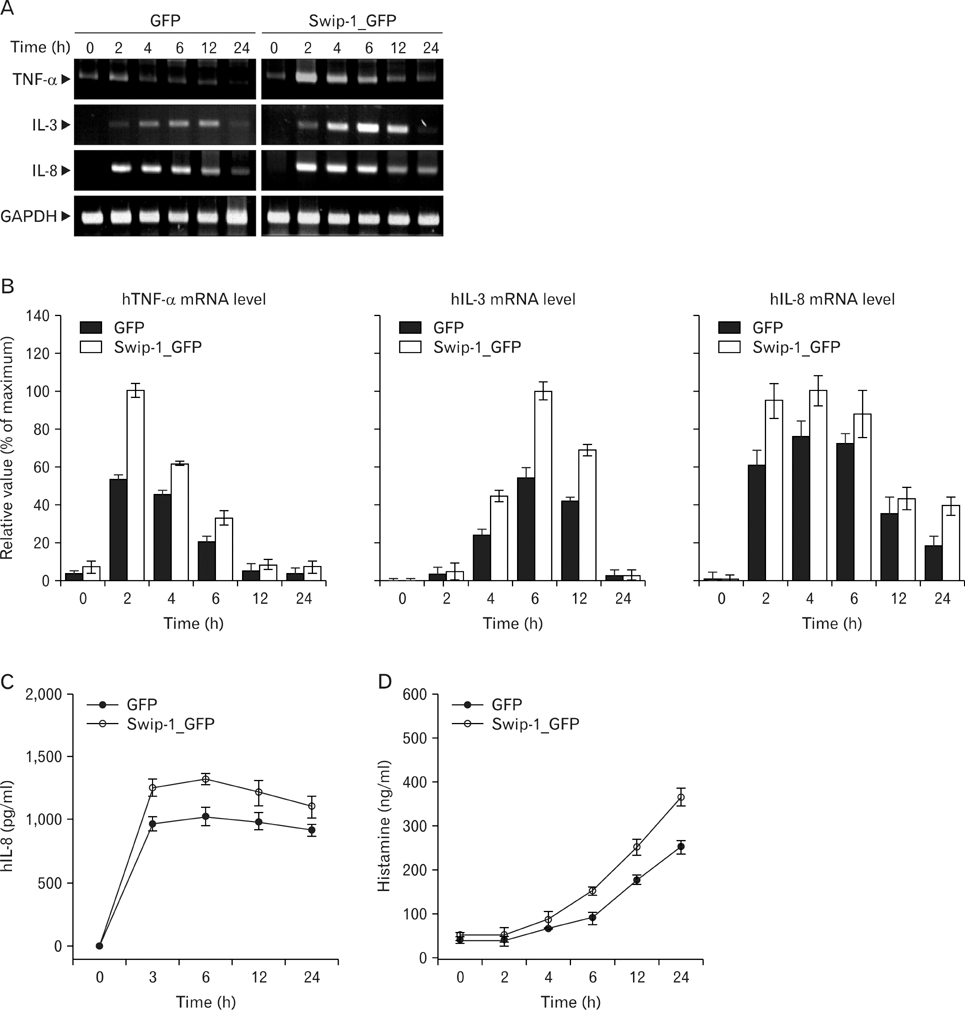
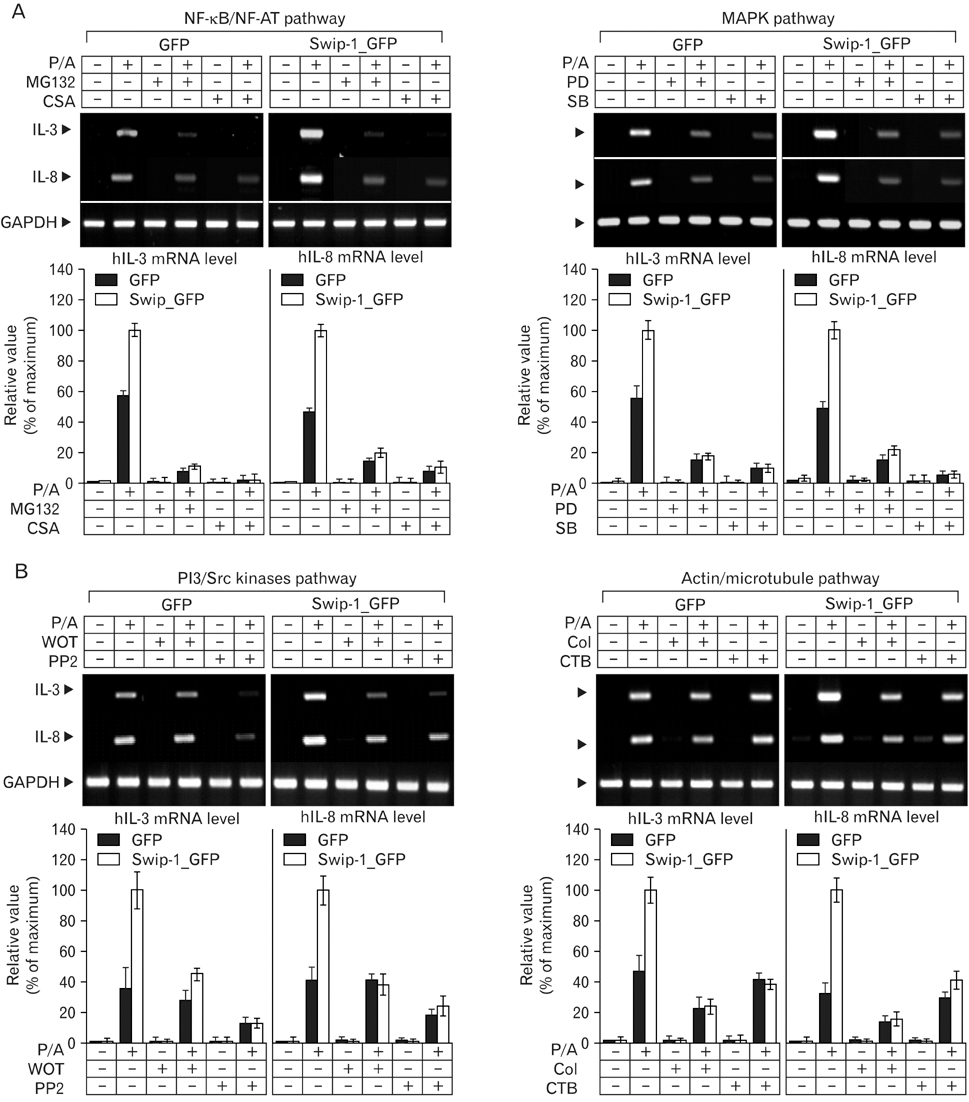
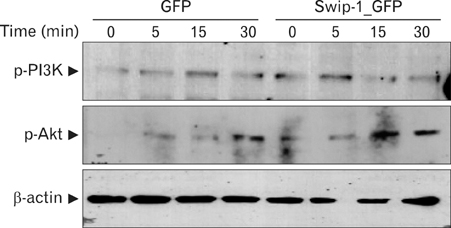
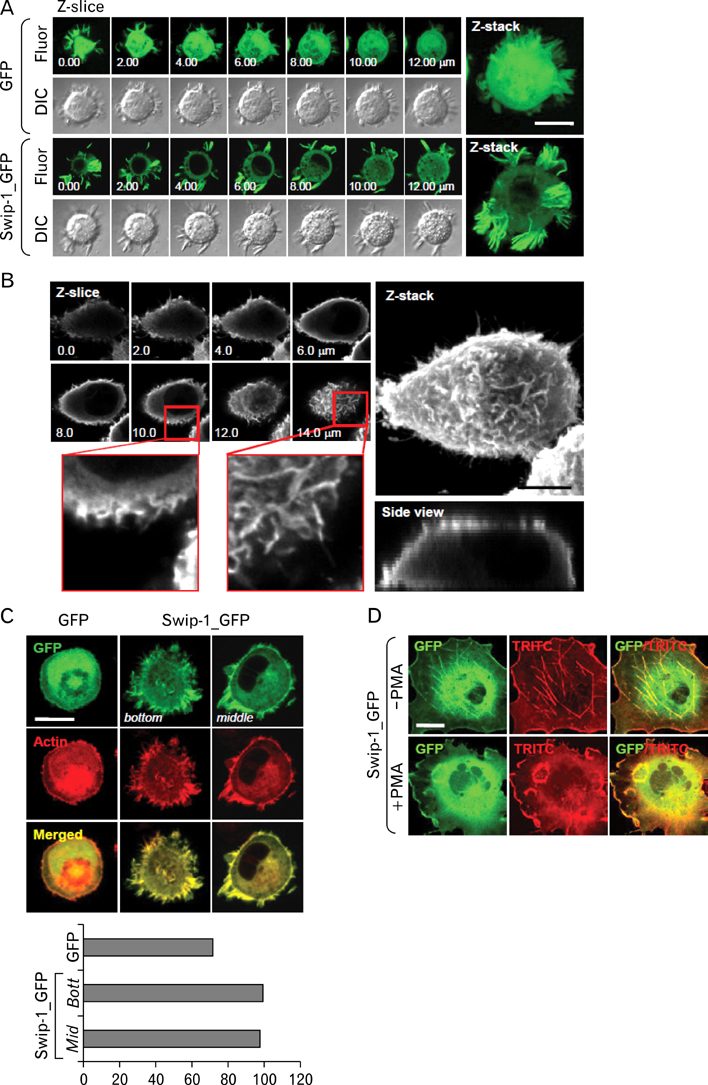
Swiprosin-1 was identified in human CD8+ lymphocytes, mature B cells and non-lymphonoid tissue. We have recently reported that swiprosin-1 is expressed in mast cells and up-regulated in both in vitro and in vivo. METHODS: The expression of cytokines and swiprosin-1 were determined by by real time PCR and conventional PCR. Pharmacological inhibitors were treated to investigate potential mechanism of swiprosin-1 in mast cell activation. Actin content was evaluated by confocal microscopy and flow cytometry. RESULTS: The swiprosin-1 augmented PMA/A23187-induced expression of cytokines and release of histamine. However, knock-down of swiprosin-1 showed only a modest effect on PMA/A23187-induced cytokine expression, suggesting that swiprosin-1 has gain-of-function characteristics. Swiprosin-1 was found in microvilli-like membrane protrusions and highly co-localized with F-actin. Importantly, either disruption of actin by cytochalasin B or inhibition of PI3 kinase, an enzyme involved in actin remodeling, by wortmannin blocked cytokine expression only in swiprosin-1-overexpressing cells. CONCLUSION: These results suggest that swiprosin-1 modulates mast cell activation potentially through actin regulation.
Keyword
MeSH Terms
-
Actins
Androstadienes
B-Lymphocytes
Cytochalasin B
Cytokines
Flow Cytometry
Histamine
Humans
Lymphocytes
Mast Cells
Membranes
Microscopy, Confocal
Phosphotransferases
Polymerase Chain Reaction
Protein Kinase C
Real-Time Polymerase Chain Reaction
Actins
Androstadienes
Cytochalasin B
Cytokines
Histamine
Phosphotransferases
Protein Kinase C
Figure
Cited by 1 articles
-
Swiprosin-1 Expression Is Up-Regulated through Protein Kinase C-θ and NF-κB Pathway in T Cells
Young-Dae Kim, Min-Sung Kwon, Bo-Ra Na, Hye-Ran Kim, Hyun-Su Lee, Chang-Duk Jun
Immune Netw. 2013;13(2):55-62. doi: 10.4110/in.2013.13.2.55.
Reference
-
1. Vuadens F, Rufer N, Kress A, Corthésy P, Schneider P, Tissot JD. Identification of swiprosin 1 in human lymphocytes. Proteomics. 2004. 4:2216–2220.
Article2. Avramidou A, Kroczek C, Lang C, Schuh W, Jäck HM, Mielenz D. The novel adaptor protein Swiprosin-1 enhances BCR signals and contributes to BCR-induced apoptosis. Cell Death Differ. 2007. 14:1936–1947.
Article3. Thylur RP, Kim YD, Kwon MS, Oh HM, Kwon HK, Kim SH, Im SH, Chun JS, Park ZY, Jun CD. Swiprosin-1 is expressed in mast cells and up-regulated through the protein kinase C beta I/eta pathway. J Cell Biochem. 2009. 108:705–715.
Article4. Mielenz D, Vettermann C, Hampel M, Lang C, Avramidou A, Karas M, Jäck HM. Lipid rafts associate with intracellular B cell receptors and exhibit a B cell stage-specific protein composition. J Immunol. 2005. 174:3508–3517.
Article5. Kemp SF, Lockey RF. Anaphylaxis: a review of causes and mechanisms. J Allergy Clin Immunol. 2002. 110:341–348.
Article6. Metzger H, Alcaraz G, Hohman R, Kinet JP, Pribluda V, Quarto R. The receptor with high affinity for immunoglobulin E. Annu Rev Immunol. 1986. 4:419–470.
Article7. Rivera J, Gilfillan AM. Molecular regulation of mast cell activation. J Allergy Clin Immunol. 2006. 117:1214–1226.
Article8. Metcalfe DD, Baram D, Mekori YA. Mast cells. Physiol Rev. 1997. 77:1033–1079.
Article9. Church MK, Levi-Schaffer F. The human mast cell. J Allergy Clin Immunol. 1997. 99:155–160.
Article10. Plaut M, Pierce JH, Watson CJ, Hanley-Hyde J, Nordan RP, Paul WE. Mast cell lines produce lymphokines in response to cross-linkage of Fc epsilon RI or to calcium ionophores. Nature. 1989. 339:64–67.
Article11. Wodnar-Filipowicz A, Heusser CH, Moroni C. Production of the haemopoietic growth factors GM-CSF and interleukin-3 by mast cells in response to IgE receptor-mediated activation. Nature. 1989. 339:150–152.
Article12. Gordon JR, Galli SJ. Mast cells as a source of both preformed and immunologically inducible TNF-alpha/cachectin. Nature. 1990. 346:274–276.
Article13. Blagoev B, Ong SE, Kratchmarova I, Mann M. Temporal analysis of phosphotyrosine-dependent signaling networks by quantitative proteomics. Nat Biotechnol. 2004. 22:1139–1145.
Article14. Marquardt DL, Walker LL. Dependence of mast cell IgE-mediated cytokine production on nuclear factor-kappaB activity. J Allergy Clin Immunol. 2000. 105:500–505.
Article15. Kaye RE, Fruman DA, Bierer BE, Albers MW, Zydowsky LD, Ho SI, Jin YJ, Castells MC, Schreiber SL, Walsh CT, et al. Effects of cyclosporin A and FK506 on Fc epsilon receptor type I-initiated increases in cytokine mRNA in mouse bone marrow-derived progenitor mast cells: resistance to FK506 is associated with a deficiency in FK506-binding protein FKBP12. Proc Natl Acad Sci U S A. 1992. 89:8542–8546.
Article16. Feoktistov I, Goldstein AE, Biaggioni I. Role of p38 mitogen-activated protein kinase and extracellular signal-regulated protein kinase kinase in adenosine A2B receptor-mediated interleukin-8 production in human mast cells. Mol Pharmacol. 1999. 55:726–734.17. Park HH, Lee S, Oh JM, Lee MS, Yoon KH, Park BH, Kim JW, Song H, Kim SH. Anti-inflammatory activity of fisetin in human mast cells (HMC-1). Pharmacol Res. 2007. 55:31–37.
Article18. Boudreau RT, Hoskin DW, Lin TJ. Phosphatase inhibition potentiates IL-6 production by mast cells in response to FcepsilonRI-mediated activation: involvement of p38 MAPK. J Leukoc Biol. 2004. 76:1075–1081.
Article19. Samayawardhena LA, Kapur R, Craig AW. Involvement of Fyn kinase in Kit and integrin-mediated Rac activation, cytoskeletal reorganization, and chemotaxis of mast cells. Blood. 2007. 109:3679–3686.
Article20. Vosseller K, Stella G, Yee NS, Besmer P. c-kit receptor signaling through its phosphatidylinositide-3'-kinase-binding site and protein kinase C: role in mast cell enhancement of degranulation, adhesion, and membrane ruffling. Mol Biol Cell. 1997. 8:909–922.
Article21. Frigeri L, Apgar JR. The role of actin microfilaments in the down-regulation of the degranulation response in RBL-2H3 mast cells. J Immunol. 1999. 162:2243–2250.22. Oka T, Hori M, Tanaka A, Matsuda H, Karaki H, Ozaki H. IgE alone-induced actin assembly modifies calcium signaling and degranulation in RBL-2H3 mast cells. Am J Physiol Cell Physiol. 2004. 286:C256–C263.
Article23. Oka T, Hori M, Ozaki H. Microtubule disruption suppresses allergic response through the inhibition of calcium influx in the mast cell degranulation pathway. J Immunol. 2005. 174:4584–4589.
Article24. Edgar AJ, Bennett JP. Circular ruffle formation in rat basophilic leukemia cells in response to antigen stimulation. Eur J Cell Biol. 1997. 73:132–140.25. Wong CK, Tsang CM, Ip WK, Lam CW. Molecular mechanisms for the release of chemokines from human leukemic mast cell line (HMC)-1 cells activated by SCF and TNF-alpha: roles of ERK, p38 MAPK, and NF-kappaB. Allergy. 2006. 61:289–297.
Article26. Rivera J, Arudchandran R, Gonzalez-Espinosa C, Manetz TS, Xirasagar S. A perspective: regulation of IgE receptor-mediated mast cell responses by a LAT-organized plasma membrane-localized signaling complex. Int Arch Allergy Immunol. 2001. 124:137–141.
Article27. Silverman MA, Shoag J, Wu J, Koretzky GA. Disruption of SLP-76 interaction with Gads inhibits dynamic clustering of SLP-76 and FcepsilonRI signaling in mast cells. Mol Cell Biol. 2006. 26:1826–1838.
Article28. Altman A, Villalba M. Protein kinase C-theta (PKCtheta): it's all about location, location, location. Immunol Rev. 2003. 192:53–63.29. Mayer BJ. SH3 domains: complexity in moderation. J Cell Sci. 2001. 114:1253–1263.
Article30. Daly RJ. Cortactin signalling and dynamic actin networks. Biochem J. 2004. 382:13–25.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Swiprosin-1 Expression Is Up-Regulated through Protein Kinase C-theta and NF-kappaB Pathway in T Cells
- Induction and Expression of Chemokines and Their Receptors in Human Mast Cell Line ( HMC - 1 )
- Involvement of MITF-A, an alternative isoform of mi transcription factor, on the expression of tryptase gene in human mast cells
- Toll‐Like Receptor 7 (TLR7) Mediated Transcriptomic Changes on Human Mast Cells
- Functional Expression of TRPV4 Cation Channels in Human Mast Cell Line (HMC-1)