Korean J Physiol Pharmacol.
2016 Jan;20(1):129-136. 10.4196/kjpp.2016.20.1.129.
The effects of intra-articular resiniferatoxin on monosodium iodoacetate-induced osteoarthritic pain in rats
- Affiliations
-
- 1Neuroscience Research Institute and Department of Physiology, Korea University College of Medicine, Seoul 02841, Korea.
- 2Rehabilitation Science Program, Department of Public Health Science, Graduate School, Korea University, Seoul 02841, Korea. junokim@korea.ac.kr
- 3School of Health and Fitness Management, College of Health and Welfare, Woosong University, Daejeon 34606, Korea.
- 4Department of Rehabilitation Policy and Standardization, National Rehabilitation Research Institute (KNRRI), Seoul 01022, Korea.
- 5Department of Physical Therapy, Korea University College of Health Science, Seoul 02841, Korea.
- 6Advanced Biomedical and Welfare Group, Korea Institute of Industrial Technology (KITECH), Cheonan 31056, Korea.
- KMID: 2150482
- DOI: http://doi.org/10.4196/kjpp.2016.20.1.129
Abstract
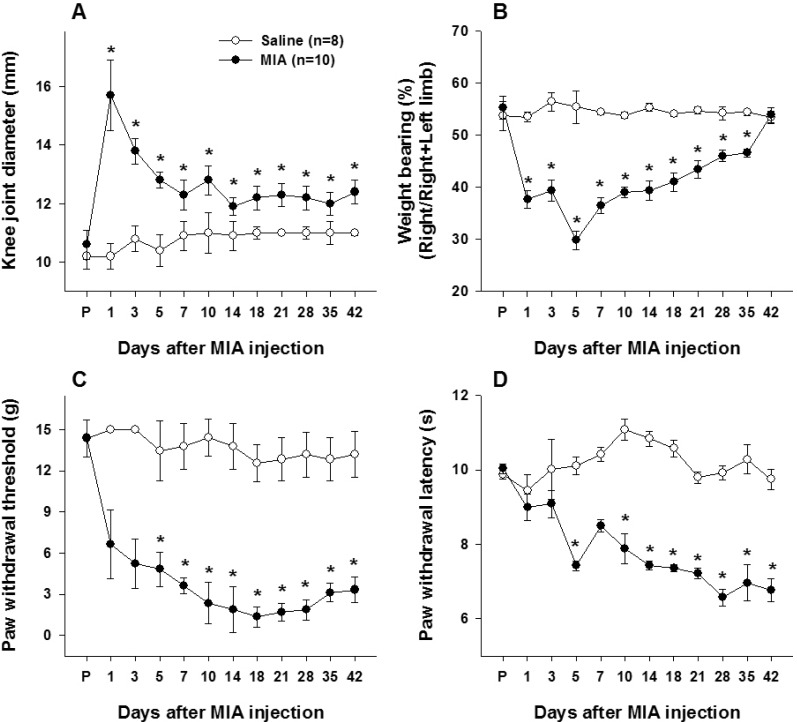
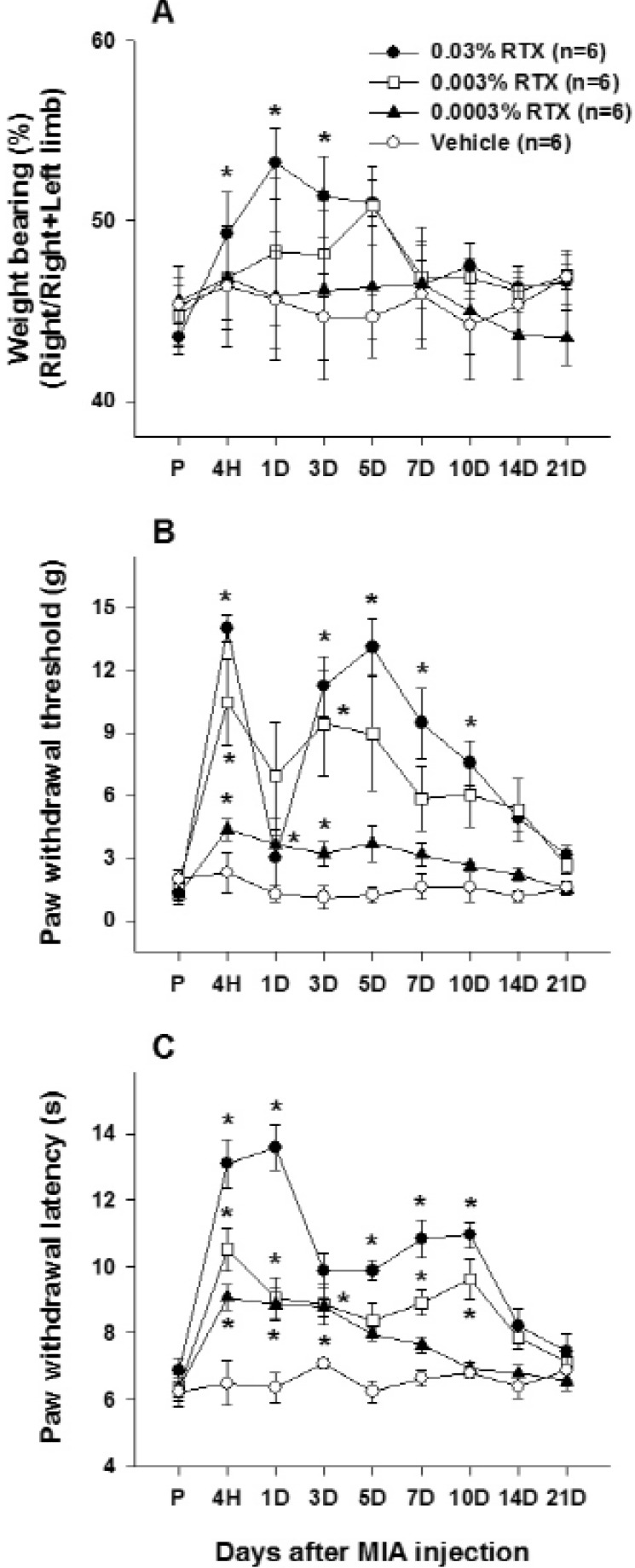
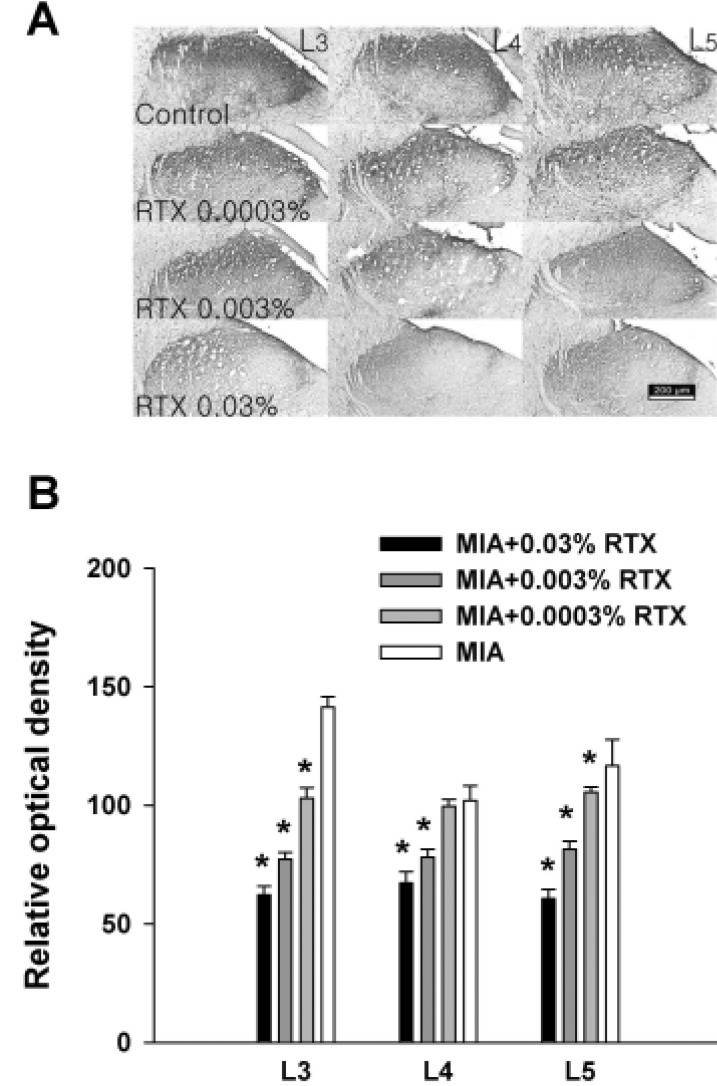
- This study was performed to investigate whether an intra-articular injection of transient receptor potential vanilloid 1 (TRPV1) receptor agonist, resiniferatoxin (RTX) would alleviate behavioral signs of arthritic pain in a rat model of osteoarthritis (OA). We also sought to determine the effect of RTX treatment on calcitonin gene-related peptide (CGRP) expression in the spinal cord. Knee joint inflammation was induced by intra-articular injection of monosodium iodoacetate (MIA, 8 mg/50 microl) and weight bearing percentage on right and left hindpaws during walking, paw withdrawal threshold to mechanical stimulation, and paw withdrawal latency to heat were measured to evaluate pain behavior. Intra-articular administration of RTX (0.03, 0.003 and 0.0003%) at 2 weeks after the induction of knee joint inflammation significantly improved reduction of weight bearing on the ipsilateral hindlimb and increased paw withdrawal sensitivity to mechanical and heat stimuli. The reduction of pain behavior persisted for 3~10 days according to each behavioral test. The MIA-induced increase in CGRP immunoreactivity in the spinal cord was decreased by RTX treatment in a dose-dependent manner. The present study demonstrated that a single intra-articular administration of RTX reduced pain behaviors for a relatively long time in an experimental model of OA and could normalize OA-associated changes in peptide expression in the spinal cord.
Keyword
MeSH Terms
Figure
Reference
-
1. Cross M, Smith E, Hoy D, Nolte S, Ackerman I, Fransen M, Bridgett L, Williams S, Guillemin F, Hill CL, Laslett LL, Jones G, Cicuttini F, Osborne R, Vos T, Buchbinder R, Woolf A, March L. The global burden of hip and knee osteoarthritis: estimates from the global burden of disease 2010 study. Ann Rheum Dis. 2014; 73:1323–1330. PMID: 24553908.
Article2. Zhang Y, Jordan JM. Epidemiology of osteoarthritis. Clin Geriatr Med. 2010; 26:355–369. PMID: 20699159.
Article3. Pritzker KP, Gay S, Jimenez SA, Ostergaard K, Pelletier JP, Revell PA, Salter D, van den Berg WB. Osteoarthritis cartilage histopathology: grading and staging. Osteoarthritis Cartilage. 2006; 14:13–29. PMID: 16242352.
Article4. Goldring MB, Goldring SR. Articular cartilage and subchondral bone in the pathogenesis of osteoarthritis. Ann N Y Acad Sci. 2010; 1192:230–237. PMID: 20392241.
Article5. Kim YM, Joo YB. Patellofemoral osteoarthritis. Knee Surg Relat Res. 2012; 24:193–200. PMID: 23269956.
Article6. Li G, Yin J, Gao J, Cheng TS, Pavlos NJ, Zhang C, Zheng MH. Subchondral bone in osteoarthritis: insight into risk factors and microstructural changes. Arthritis Res Ther. 2013; 15:223. PMID: 24321104.
Article7. Tchetina EV. Developmental mechanisms in articular cartilage degradation in osteoarthritis. Arthritis. 2011; 2011:683970. PMID: 22046522.
Article8. Thakur M, Dickenson AH, Baron R. Osteoarthritis pain: nociceptive or neuropathic? Nat Rev Rheumatol. 2014; 10:374–380. PMID: 24686507.
Article9. Peppin JF, Pappagallo M. Capsaicinoids in the treatment of neuropathic pain: a review. Ther Adv Neurol Disord. 2014; 7:22–32. PMID: 24409200.
Article10. Ahern GP, Brooks IM, Miyares RL, Wang XB. Extracellular cations sensitize and gate capsaicin receptor TRPV1 modulating pain signaling. J Neurosci. 2005; 25:5109–5116. PMID: 15917451.
Article11. Karai L, Brown DC, Mannes AJ, Connelly ST, Brown J, Gandal M, Wellisch OM, Neubert JK, Olah Z, Iadarola MJ. Deletion of vanilloid receptor 1-expressing primary afferent neurons for pain control. J Clin Invest. 2004; 113:1344–1352. PMID: 15124026.
Article12. Fernihough J, Gentry C, Bevan S, Winter J. Regulation of calcitonin gene-related peptide and TRPV1 in a rat model of osteoarthritis. Neurosci Lett. 2005; 388:75–80. PMID: 16039054.
Article13. Kelly S, Chapman RJ, Woodhams S, Sagar DR, Turner J, Burston JJ, Bullock C, Paton K, Huang J, Wong A, McWilliams DF, Okine BN, Barrett DA, Hathway GJ, Walsh DA, Chapman V. Increased function of pronociceptive TRPV1 at the level of the joint in a rat model of osteoarthritis pain. Ann Rheum Dis. 2015; 74:252–259. PMID: 24152419.
Article14. Longo G, Osikowicz M, Ribeiro-da-Silva A. Sympathetic fiber sprouting in inflamed joints and adjacent skin contributes to painrelated behavior in arthritis. J Neurosci. 2013; 33:10066–10074. PMID: 23761902.
Article15. Cui M, Honore P, Zhong C, Gauvin D, Mikusa J, Hernandez G, Chandran P, Gomtsyan A, Brown B, Bayburt EK, Marsh K, Bianchi B, McDonald H, Niforatos W, Neelands TR, Moreland RB, Decker MW, Lee CH, Sullivan JP, Faltynek CR. TRPV1 receptors in the CNS play a key role in broad-spectrum analgesia of TRPV1 antagonists. J Neurosci. 2006; 26:9385–9393. PMID: 16971522.
Article16. Gavva NR, Treanor JJ, Garami A, Fang L, Surapaneni S, Akrami A, Alvarez F, Bak A, Darling M, Gore A, Jang GR, Kesslak JP, Ni L, Norman MH, Palluconi G, Rose MJ, Salfi M, Tan E, Romanovsky AA, Banfield C, Davar G. Pharmacological blockade of the vanilloid receptor TRPV1 elicits marked hyperthermia in humans. Pain. 2008; 136:202–210. PMID: 18337008.
Article17. Abdelhamid RE, Kovács KJ, Honda CN, Nunez MG, Larson AA. Resiniferatoxin (RTX) causes a uniquely protracted musculoskeletal hyperalgesia in mice by activation of TRPV1 receptors. J Pain. 2013; 14:1629–1641. PMID: 24188863.
Article18. Kissin EY, Freitas CF, Kissin I. The effects of intraarticular resiniferatoxin in experimental knee-joint arthritis. Anesth Analg. 2005; 101:1433–1439. PMID: 16244007.
Article19. Min SS, Han JS, Kim YI, Na HS, Yoon YW, Hong SK, Han HC. A novel method for convenient assessment of arthritic pain in voluntarily walking rats. Neurosci Lett. 2001; 308:95–98. PMID: 11457568.
Article20. Chaplan SR, Malmberg AB, Yaksh TL. Efficacy of spinal NMDA receptor antagonism in formalin hyperalgesia and nerve injury evoked allodynia in the rat. J Pharmacol Exp Ther. 1997; 280:829–838. PMID: 9023297.21. Dixon WJ. Efficient analysis of experimental observations. Annu Rev Pharmacol Toxicol. 1980; 20:441–462. PMID: 7387124.
Article22. Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988; 32:77–88. PMID: 3340425.
Article23. Lee KS, Kim J, Yoon YW, Lee MG, Hong SK, Han HC. The peripheral role of group I metabotropic glutamate receptors on nociceptive behaviors in rats with knee joint inflammation. Neurosci Lett. 2007; 416:123–127. PMID: 17314010.
Article24. Neugebauer V, Han JS, Adwanikar H, Fu Y, Ji G. Techniques for assessing knee joint pain in arthritis. Mol Pain. 2007; 3:8. PMID: 17391515.
Article25. Guzman RE, Evans MG, Bove S, Morenko B, Kilgore K. Monoiodoacetate-induced histologic changes in subchondral bone and articular cartilage of rat femorotibial joints: an animal model of osteoarthritis. Toxicol Pathol. 2003; 31:619–624. PMID: 14585729.
Article26. Orita S, Ishikawa T, Miyagi M, Ochiai N, Inoue G, Eguchi Y, Kamoda H, Arai G, Toyone T, Aoki Y, Kubo T, Takahashi K, Ohtori S. Pain-related sensory innervation in monoiodoacetate-induced osteoarthritis in rat knees that gradually develops neuronal injury in addition to inflammatory pain. BMC Musculoskelet Disord. 2011; 12:134. PMID: 21679434.27. van der Kraan PM, Vitters EL, van de Putte LB, van den Berg WB. Development of osteoarthritic lesions in mice by "metabolic" and "mechanical" alterations in the knee joints. Am J Pathol. 1989; 135:1001–1014. PMID: 2556924.28. Cady RJ, Glenn JR, Smith KM, Durham PL. Calcitonin gene-related peptide promotes cellular changes in trigeminal neurons and glia implicated in peripheral and central sensitization. Mol Pain. 2011; 7:94. PMID: 22145886.
Article29. Neugebauer V, Schaible HG. Evidence for a central component in the sensitization of spinal neurons with joint input during development of acute arthritis in cat's knee. J Neurophysiol. 1990; 64:299–311. PMID: 2388073.
Article30. Liu P, Okun A, Ren J, Guo RC, Ossipov MH, Xie J, King T, Porreca F. Ongoing pain in the MIA model of osteoarthritis. Neurosci Lett. 2011; 493:72–75. PMID: 21241772.
Article31. Ferreira-Gomes J, Adães S, Sousa RM, Mendonça M, Castro-Lopes JM. Dose-dependent expression of neuronal injury markers during experimental osteoarthritis induced by monoiodoacetate in the rat. Mol Pain. 2012; 8:50. PMID: 22769424.
Article32. Hirasawa Y, Okajima S, Ohta M, Tokioka T. Nerve distribution to the human knee joint: anatomical and immunohistochemical study. Int Orthop. 2000; 24:1–4. PMID: 10774852.
Article33. Widenfalk B, Wiberg M. Origin of sympathetic and sensory innervation of the knee joint. A retrograde axonal tracing study in the rat. Anat Embryol (Berl). 1989; 180:317–323. PMID: 2478046.34. Felson DT, Chaisson CE, Hill CL, Totterman SM, Gale ME, Skinner KM, Kazis L, Gale DR. The association of bone marrow lesions with pain in knee osteoarthritis. Ann Intern Med. 2001; 134:541–549. PMID: 11281736.
Article35. Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997; 389:816–824. PMID: 9349813.
Article36. Tominaga M, Caterina MJ, Malmberg AB, Rosen TA, Gilbert H, Skinner K, Raumann BE, Basbaum AI, Julius D. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron. 1998; 21:531–543. PMID: 9768840.
Article37. Murakami K, Nakagawa H, Nishimura K, Matsuo S. Changes in peptidergic fiber density in the synovium of mice with collagenaseinduced acute arthritis. Can J Physiol Pharmacol. 2015; 93:435–441. PMID: 25909759.
Article38. Knotkova H, Pappagallo M, Szallasi A. Capsaicin (TRPV1 Agonist) therapy for pain relief: farewell or revival? Clin J Pain. 2008; 24:142–154. PMID: 18209521.39. Szallasi A, Blumberg PM. Vanilloid (Capsaicin) receptors and mechanisms. Pharmacol Rev. 1999; 51:159–212. PMID: 10353985.40. Wendler J, Burmester GR, Sörensen H, Krause A, Richter C, Tony HP, Rubbert-Roth A, Bartz-Bazzanella P, Wassenberg S, Haug-Rost I, Dörner T. Rituximab in patients with rheumatoid arthritis in routine practice (GERINIS): six-year results from a prospective, multicentre, non-interventional study in 2,484 patients. Arthritis Res Ther. 2014; 16:R80. PMID: 24670196.
Article41. Ferland CE, Pailleux F, Vachon P, Beaudry F. Determination of specific neuropeptides modulation time course in a rat model of osteoarthritis pain by liquid chromatography ion trap mass spectrometry. Neuropeptides. 2011; 45:423–429. PMID: 21855139.
Article42. Puttfarcken PS, Han P, Joshi SK, Neelands TR, Gauvin DM, Baker SJ, Lewis LG, Bianchi BR, Mikusa JP, Koenig JR, Perner RJ, Kort ME, Honore P, Faltynek CR, Kym PR, Reilly RM. A-995662 [(R)-8-(4-methyl-5-(4-(trifluoromethyl)phenyl)oxazol-2-ylamino)-1,2,3,4-tetrahydronaphthalen-2-ol], a novel, selective TRPV1 receptor antagonist, reduces spinal release of glutamate and CGRP in a rat knee joint pain model. Pain. 2010; 150:319–326. PMID: 20621685.
Article43. Meng J, Ovsepian SV, Wang J, Pickering M, Sasse A, Aoki KR, Lawrence GW, Dolly JO. Activation of TRPV1 mediates calcitonin gene-related peptide release, which excites trigeminal sensory neurons and is attenuated by a retargeted botulinum toxin with anti-nociceptive potential. J Neurosci. 2009; 29:4981–4992. PMID: 19369567.
Article44. Brown DC, Agnello K, Iadarola MJ. Intrathecal resiniferatoxin in a dog model: efficacy in bone cancer pain. Pain. 2015; 156:1018–1024. PMID: 25659068.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Anti-osteoarthritis effect of Boswellia serrata gum resin extract in monosodium iodoacetate-induced osteoarthritic Sprague-Dawley rats
- Anti-inflammatory effect of egg white-chalcanthite and purple bamboo salts mixture on arthritis induced by monosodium iodoacetate in Sprague-Dawley rats
- Micro-CT Arthrographic Analysis of Monosodium Iodoacetate-Induced Osteoarthritis in Rat Knees
- Pathological Characteristics of Monosodium Iodoacetate-Induced Osteoarthritis in Rats
- Ursodeoxycholic Acid Ameliorates Pain Severity and Cartilage Degeneration in Monosodium Iodoacetate-Induced Osteoarthritis in Rats