Korean J Physiol Pharmacol.
2016 Jan;20(1):53-62. 10.4196/kjpp.2016.20.1.53.
Effects of CoCl2 on multi-lineage differentiation of C3H/10T1/2 mesenchymal stem cells
- Affiliations
-
- 1Department of Oral Anatomy, School of Dentistry, Chonnam National University, Gwangju 61186, Korea. greatone@chonnam.ac.kr
- 2Department of Dental Hygiene, Chodang University, Muan 58530, Korea.
- KMID: 2150473
- DOI: http://doi.org/10.4196/kjpp.2016.20.1.53
Abstract
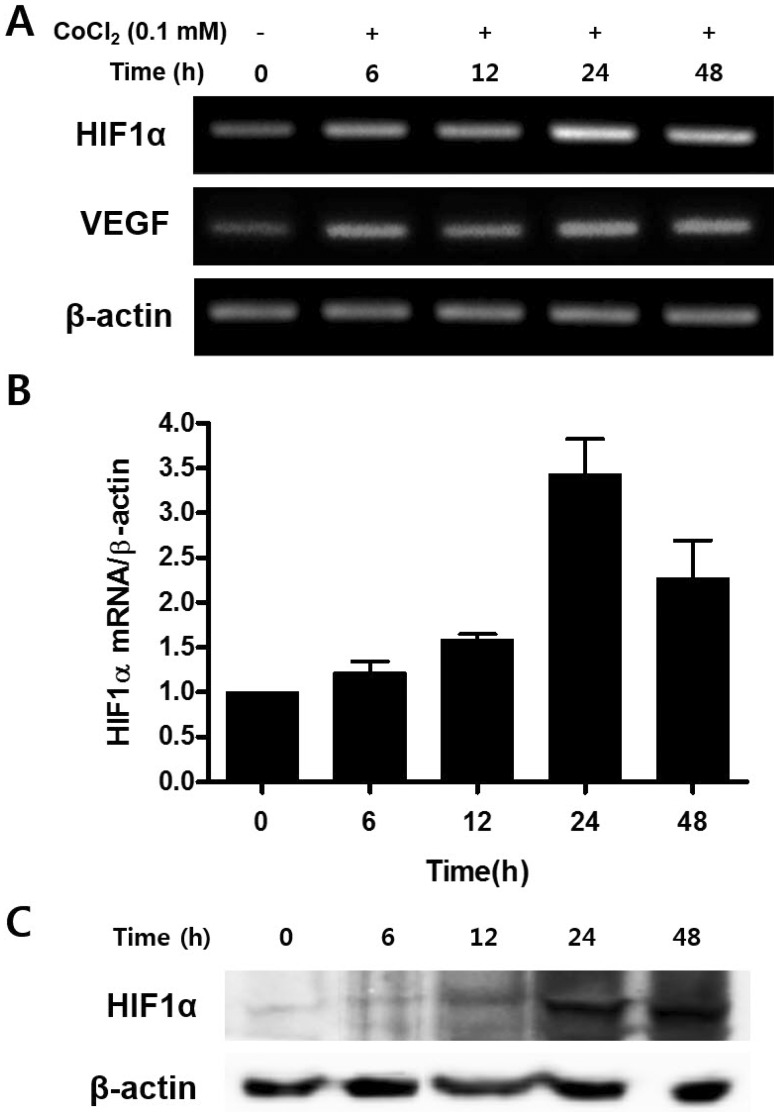
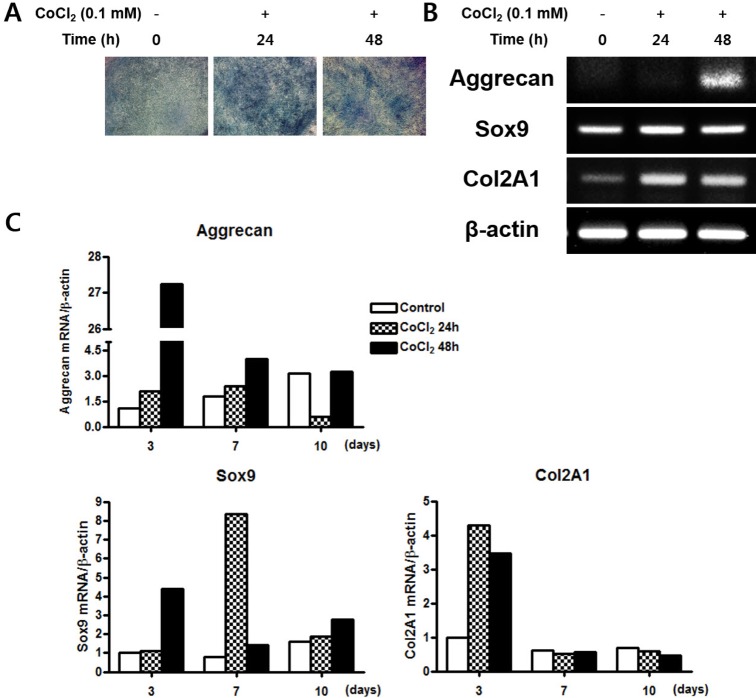
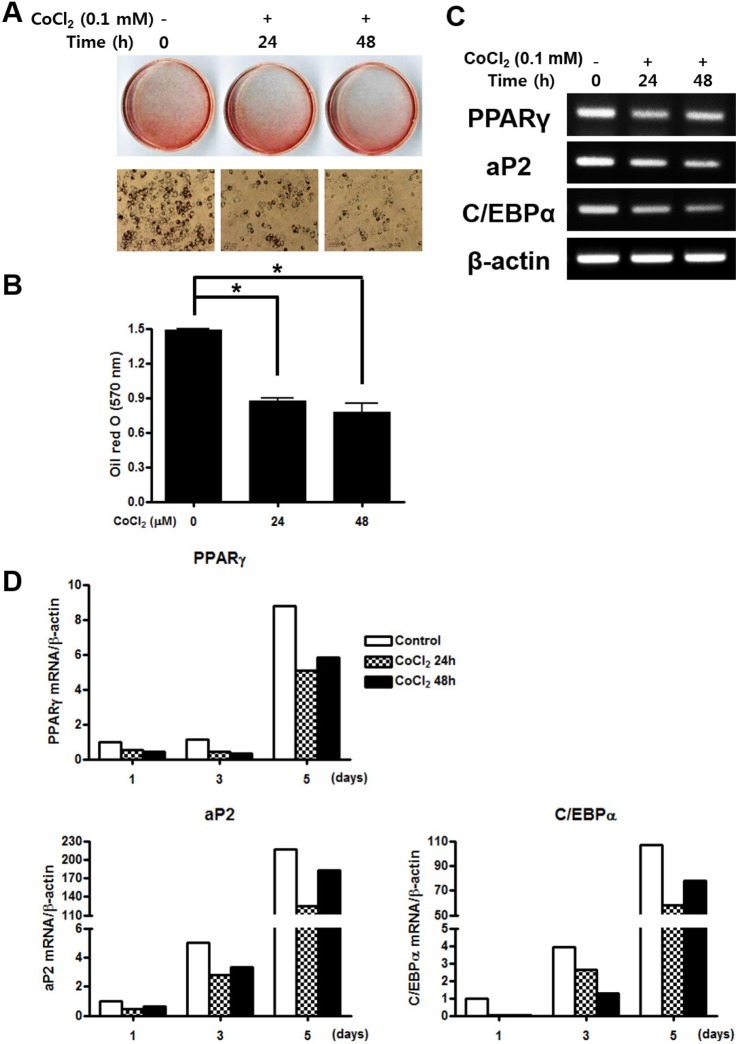
- Mesenchymal stem cells (MSCs) in the bone marrow and other somatic tissues reside in an environment with relative low oxygen tension. Cobalt chloride (CoCl2) can mimic hypoxic conditions through transcriptional changes of some genes including hypoxia-inducible factor-1alpha (HIF-1alpha) and vascular endothelial growth factor (VEGF). This study evaluated the potential role of CoCl2 preconditioning on multi-lineage differentiation of C3H/10T1/2, a murine MSC line to understand its possible molecular mechanisms in vitro. CoCl2 treatment of MSCs markedly increased HIF-1alpha and VEGF mRNA, and protein expression of HIF-1alpha. Temporary preconditioning of MSCs with CoCl2 induced up-regulation of osteogenic markers including alkaline phosphatase, osteocalcin, and type I collagen during osteogenic differentiation, followed by enhanced mineralization. CoCl2 also increased chondrogenic markers including aggrecan, sox9, and type II collagen, and promoted chondrocyte differentiation. CoCl2 suppressed the expression of adipogenic markers including PPARgamma, aP2, and C/EBPalpha, and inhibited adipogenesis. Temporary preconditioning with CoCl2 could affect the multi-lineage differentiation of MSCs.
Keyword
MeSH Terms
-
Adipogenesis
Aggrecans
Alkaline Phosphatase
Anoxia
Bone Marrow
Chondrocytes
Cobalt
Collagen Type I
Collagen Type II
Mesenchymal Stromal Cells*
Osteocalcin
Oxygen
PPAR gamma
RNA, Messenger
Up-Regulation
Vascular Endothelial Growth Factor A
Aggrecans
Alkaline Phosphatase
Cobalt
Collagen Type I
Collagen Type II
Osteocalcin
Oxygen
PPAR gamma
RNA, Messenger
Vascular Endothelial Growth Factor A
Figure
Reference
-
1. Baksh D, Song L, Tuan RS. Adult mesenchymal stem cells: characterization, differentiation, and application in cell and gene therapy. J Cell Mol Med. 2004; 8:301–316. PMID: 15491506.
Article2. Tsai CC, Chen YJ, Yew TL, Chen LL, Wang JY, Chiu CH, Hung SC. Hypoxia inhibits senescence and maintains mesenchymal stem cell properties through down-regulation of E2A-p21 by HIF-TWIST. Blood. 2011; 117:459–469. PMID: 20952688.
Article3. Bruder SP, Fink DJ, Caplan AI. Mesenchymal stem cells in bone development, bone repair, and skeletal regeneration therapy. J Cell Biochem. 1994; 56:283–294. PMID: 7876320.4. Cicione C, Muiños-López E, Hermida-Gómez T, Fuentes- Boquete I, Díaz-Prado S, Blanco FJ, et al. Effects of severe hypoxia on bone marrow mesenchymal stem cells differentiation potential. Stem Cells Int. 2013; 2013:232896. PMID: 24082888.
Article5. Ren H, Cao Y, Zhao Q, Li J, Zhou C, Liao L, Jia M, Zhao Q, Cai H, Han ZC, Yang R, Chen G, Zhao RC. Proliferation and differentiation of bone marrow stromal cells under hypoxic conditions. Biochem Biophys Res Commun. 2006; 347:12–21. PMID: 16814746.
Article6. Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999; 284:143–147. PMID: 10102814.
Article7. Rosenbaum AJ, Grande DA, Dines JS. The use of mesenchymal stem cells in tissue engineering: A global assessment. Organogenesis. 2008; 4:23–27. PMID: 19279711.8. Tsai CC, Yew TL, Yang DC, Huang WH, Hung SC. Benefits of hypoxic culture on bone marrow multipotent stromal cells. Am J Blood Res. 2012; 2:148–159. PMID: 23119226.9. Das R, Jahr H, van Osch GJ, Farrell E. The role of hypoxia in bone marrow-derived mesenchymal stem cells: considerations for regenerative medicine approaches. Tissue Eng Part B Rev. 2010; 16:159–168. PMID: 19698058.
Article10. Lee HH, Chang CC, Shieh MJ, Wang JP, Chen YT, Young TH, Hung SC. Hypoxia enhances chondrogenesis and prevents terminal differentiation through PI3K/Akt/FoxO dependent anti-apoptotic effect. Sci Rep. 2013; 3:2683. PMID: 24042188.
Article11. Ercan E, Bagla AG, Aksoy A, Gacar G, Unal ZS, Asgun HF, Karaoz E. In vitro protection of adipose tissue-derived mesenchymal stem cells by erythropoietin. Acta Histochem. 2014; 116:117–125. PMID: 24011510.
Article12. Satija NK, Singh VK, Verma YK, Gupta P, Sharma S, Afrin F, Sharma M, Sharma P, Tripathi RP, Gurudutta GU. Mesenchymal stem cell-based therapy: a new paradigm in regenerative medicine. J Cell Mol Med. 2009; 13:4385–4402. PMID: 19602034.
Article13. Müller J, Benz K, Ahlers M, Gaissmaier C, Mollenhauer J. Hypoxic conditions during expansion culture prime human mesenchymal stromal precursor cells for chondrogenic differentiation in three-dimensional cultures. Cell Transplant. 2011; 20:1589–1602. PMID: 21396167.
Article14. Wagegg M, Gaber T, Lohanatha FL, Hahne M, Strehl C, Fangradt M, Tran CL, Schönbeck K, Hoff P, Ode A, Perka C, Duda GN, Buttgereit F. Hypoxia promotes osteogenesis but suppresses adipogenesis of human mesenchymal stromal cells in a hypoxia-inducible factor-1 dependent manner. PLoS One. 2012; 7:e46483. PMID: 23029528.
Article15. Holzwarth C, Vaegler M, Gieseke F, Pfister SM, Handgretinger R, Kerst G, Müller I. Low physiologic oxygen tensions reduce proliferation and differentiation of human multipotent mesenchymal stromal cells. BMC Cell Biol. 2010; 11:11. PMID: 20109207.
Article16. Duval E, Baugé C, Andriamanalijaona R, Bénateau H, Leclercq S, Dutoit S, Poulain L, Galéra P, Boumédiene K. Molecular mechanism of hypoxia-induced chondrogenesis and its application in in vivo cartilage tissue engineering. Biomaterials. 2012; 33:6042–6051. PMID: 22677190.17. Lafont JE, Talma S, Murphy CL. Hypoxia-inducible factor 2alpha is essential for hypoxic induction of the human articular chondrocyte phenotype. Arthritis Rheum. 2007; 56:3297–3306. PMID: 17907154.18. Park IH, Kim KH, Choi HK, Shim JS, Whang SY, Hahn SJ, Kwon OJ, Oh IH. Constitutive stabilization of hypoxia- inducible factor alpha selectively promotes the self-renewal of mesenchymal progenitors and maintains mesenchymal stromal cells in an undifferentiated state. Exp Mol Med. 2013; 45:e44. PMID: 24071737.19. Csete M. Oxygen in the cultivation of stem cells. Ann N Y Acad Sci. 2005; 1049:1–8.
Article20. Yang SJ, Pyen J, Lee I, Lee H, Kim Y, Kim T. Cobalt chloride-induced apoptosis and extracellular signal-regulated protein kinase 1/2 activation in rat C6 glioma cells. J Biochem Mol Biol. 2004; 37:480–486. PMID: 15469737.
Article21. Malda J, Klein TJ, Upton Z. The roles of hypoxia in the in vitro engineering of tissues. Tissue Eng. 2007; 13:2153–2162. PMID: 17516855.22. Badr GA, Zhang JZ, Tang J, Kern TS, Ismail-Beigi F. Glut1 and glut3 expression, but not capillary density, is increased by cobalt chloride in rat cerebrum and retina. Brain Res Mol Brain Res. 1999; 64:24–33. PMID: 9889305.
Article23. Wang GL, Semenza GL. Desferrioxamine induces erythropoietin gene expression and hypoxia-inducible factor 1 DNA-binding activity: implications for models of hypoxia signal transduction. Blood. 1993; 82:3610–3615. PMID: 8260699.24. Yuan Y, Hilliard G, Ferguson T, Millhorn DE. Cobalt inhibits the interaction between hypoxia-inducible factor-alpha and von Hippel-Lindau protein by direct binding to hypoxia-inducible factor-alpha. J Biol Chem. 2003; 278:15911–15916. PMID: 12606543.25. Cipolleschi MG, Dello Sbarba P, Olivotto M. The role of hypoxia in the maintenance of hematopoietic stem cells. Blood. 1993; 82:2031–2037. PMID: 8104535.
Article26. Ma T, Grayson WL, Fröhlich M, Vunjak-Novakovic G. Hypoxia and stem cell-based engineering of mesenchymal tissues. Biotechnol Prog. 2009; 25:32–42. PMID: 19198002.
Article27. Meijer GJ, de Bruijn JD, Koole R, van Blitterswijk CA. Cell based bone tissue engineering in jaw defects. Biomaterials. 2008; 29:3053–3061. PMID: 18433864.
Article28. Tang YL, Tang Y, Zhang YC, Qian K, Shen L, Phillips MI. Improved graft mesenchymal stem cell survival in ischemic heart with a hypoxia-regulated heme oxygenase-1 vector. J Am Coll Cardiol. 2005; 46:1339–1350. PMID: 16198853.
Article29. Nesselmann C, Ma N, Bieback K, Wagner W, Ho A, Konttinen YT, Zhang H, Hinescu ME, Steinhoff G. Mesenchymal stem cells and cardiac repair. J Cell Mol Med. 2008; 12:1795–1810. PMID: 18684237.
Article30. Park SK, Dadak AM, Haase VH, Fontana L, Giaccia AJ, Johnson RS. Hypoxia-induced gene expression occurs solely through the action of hypoxia-inducible factor 1alpha (HIF-1alpha): role of cytoplasmic trapping of HIF-2alpha. Mol Cell Biol. 2003; 23:4959–4971. PMID: 12832481.31. Mohyeldin A, Garzón-Muvdi T, Quiñones-Hinojosa A. Oxygen in stem cell biology: a critical component of the stem cell niche. Cell Stem Cell. 2010; 7:150–161. PMID: 20682444.
Article32. Rosenberger C, Mandriota S, Jürgensen JS, Wiesener MS, Hörstrup JH, Frei U, Ratcliffe PJ, Maxwell PH, Bachmann S, Eckardt KU, et al. Expression of hypoxia-inducible factor-1alpha and -2alpha in hypoxic and ischemic rat kidneys. J Am Soc Nephrol. 2002; 13:1721–1732. PMID: 12089367.33. Ke Q, Costa M. Hypoxia-inducible factor-1 (HIF-1). Mol Pharmacol. 2006; 70:1469–1480. PMID: 16887934.
Article34. Steinbrech DS, Mehrara BJ, Saadeh PB, Chin G, Dudziak ME, Gerrets RP, Gittes GK, Longaker MT. Hypoxia regulates VEGF expression and cellular proliferation by osteoblasts in vitro. Plast Reconstr Surg. 1999; 104:738–747. PMID: 10456527.
Article35. Kanczler JM, Oreffo RO. Osteogenesis and angiogenesis: the potential for engineering bone. Eur Cell Mater. 2008; 15:100–114. PMID: 18454418.
Article36. Lennon DP, Edmison JM, Caplan AI. Cultivation of rat marrow-derived mesenchymal stem cells in reduced oxygen tension: effects on in vitro and in vivo osteochondrogenesis. J Cell Physiol. 2001; 187:345–355. PMID: 11319758.
Article37. Robins JC, Akeno N, Mukherjee A, Dalal RR, Aronow BJ, Koopman P, Clemens TL. Hypoxia induces chondrocyte-specific gene expression in mesenchymal cells in association with transcriptional activation of Sox9. Bone. 2005; 37:313–322. PMID: 16023419.
Article38. Kanichai M, Ferguson D, Prendergast PJ, Campbell VA. Hypoxia promotes chondrogenesis in rat mesenchymal stem cells: a role for AKT and hypoxia-inducible factor (HIF)-1alpha. J Cell Physiol. 2008; 216:708–715. PMID: 18366089.39. Valorani MG, Germani A, Otto WR, Harper L, Biddle A, Khoo CP, Lin WR, Hawa MI, Tropel P, Patrizi MP, Pozzilli P, Alison MR. Hypoxia increases Sca-1/CD44 co-expression in murine mesenchymal stem cells and enhances their adipogenic differentiation potential. Cell Tissue Res. 2010; 341:111–120. PMID: 20496083.
Article40. Song JK, Lee CH, Hwang SM, Joo BS, Lee SY, Jung JS. Effect of phorbol 12-myristate 13-acetate on the differentiation of adipose-derived stromal cells from different subcutaneous adipose tissue depots. Korean J Physiol Pharmacol. 2014; 18:289–296. PMID: 25177160.
Article41. Crisostomo PR, Wang Y, Markel TA, Wang M, Lahm T, Meldrum DR. Human mesenchymal stem cells stimulated by TNF-alpha, LPS, or hypoxia produce growth factors by an NF kappa B- but not JNK-dependent mechanism. Am J Physiol Cell Physiol. 2008; 294:C675–C682. PMID: 18234850.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of CoCl2 on Osteogenic Differentiation of Human Mesenchymal Stem Cells
- Isolation and characterization of equine amniotic membrane-derived mesenchymal stem cells
- Effects of nanoscale ridge/groovepattern arrayed surface on in vitro differentiation of multi-potent pulp cells derived from human supernumerary teeth
- Survival and Graft versus Host Disease in Murine MHC Mismatched Hematopoietic Stem Cell Transplantation with Co-injection of Mesenchymal Stem Cells
- Differential Potential of Stem Cells Following Their Origin: Subacromial Bursa, Bone Marrow, Umbilical Cord Blood