Korean J Physiol Pharmacol.
2016 Jan;20(1):41-51. 10.4196/kjpp.2016.20.1.41.
Epigallocatechin-3-gallate rescues LPS-impaired adult hippocampal neurogenesis through suppressing the TLR4-NF-kappaB signaling pathway in mice
- Affiliations
-
- 1Dental Science Research Institute, School of Dentistry, Chonnam National University, Gwangju 61186, Korea. wjkim@jnu.ac.kr, jjy@jnu.ac.kr
- 2Medical Research Center for Biomineralization Disorders, School of Dentistry, Chonnam National University, Gwangju 61186, Korea.
- 3Department of Oral Physiology, School of Dentistry, Chonnam National University, Gwangju 61186, Korea.
- 4Department of Oral and Maxillofacial Surgery, School of Dentistry, Chonnam National University, Gwangju 61186, Korea.
- 5Department of Microbiology, Collage of Medicine, Seonam Universtity, Namwon 55724, Korea.
- KMID: 2150472
- DOI: http://doi.org/10.4196/kjpp.2016.20.1.41
Abstract
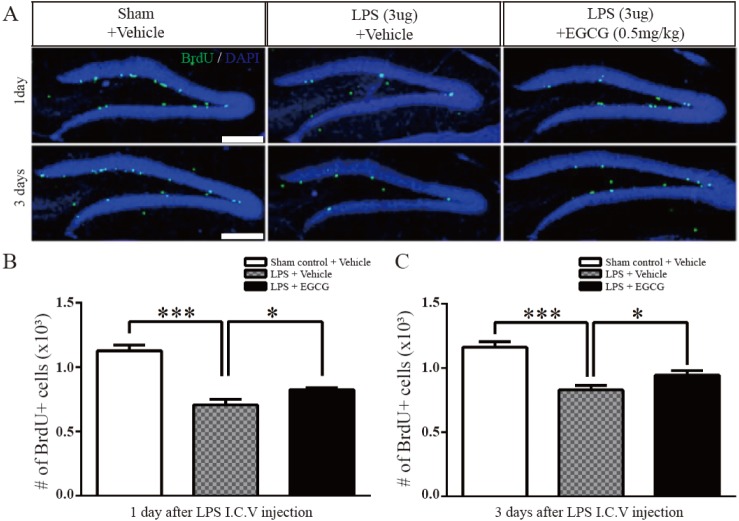
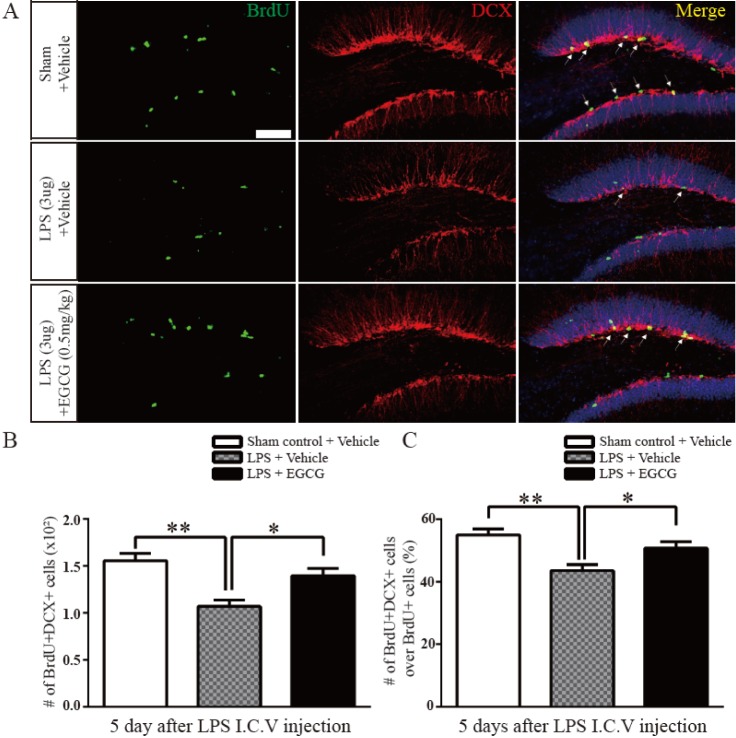
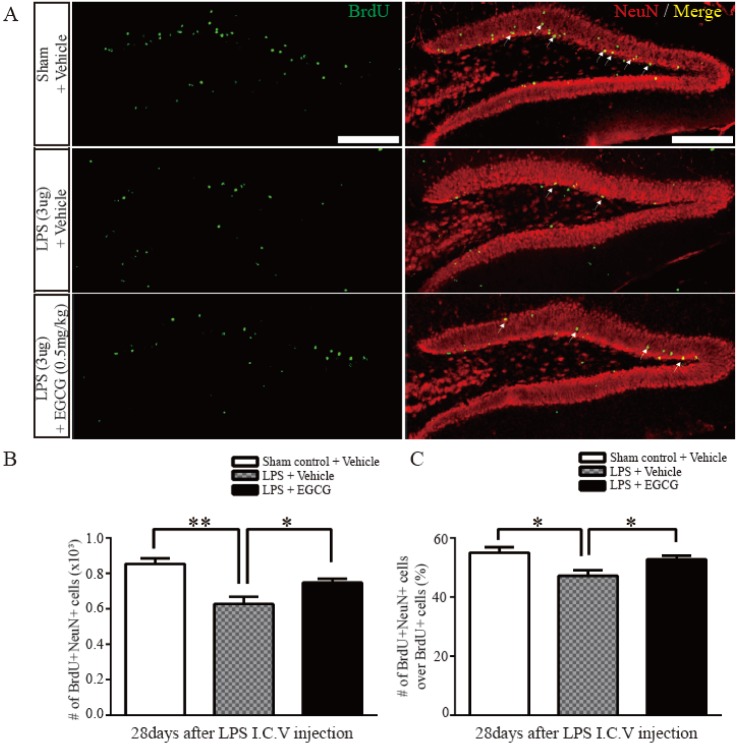
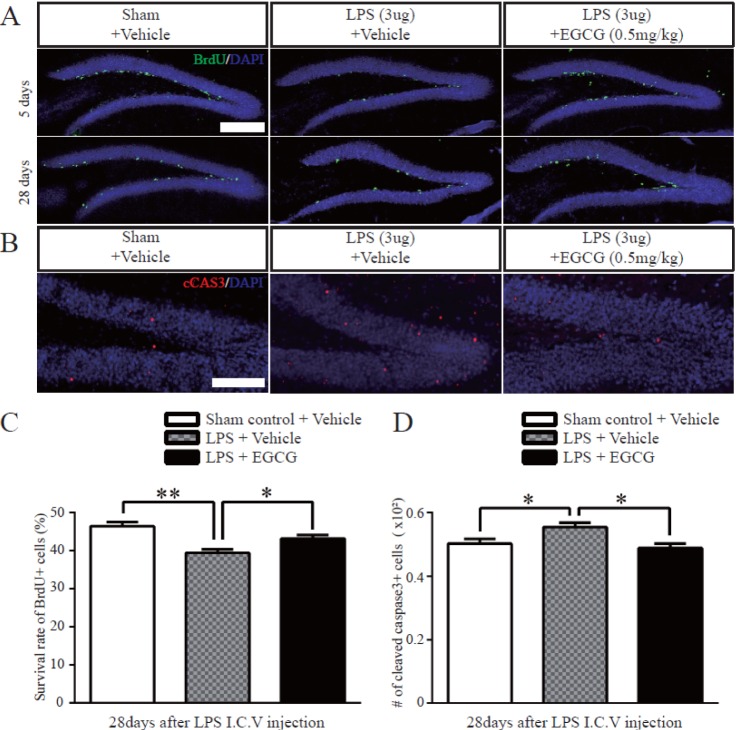
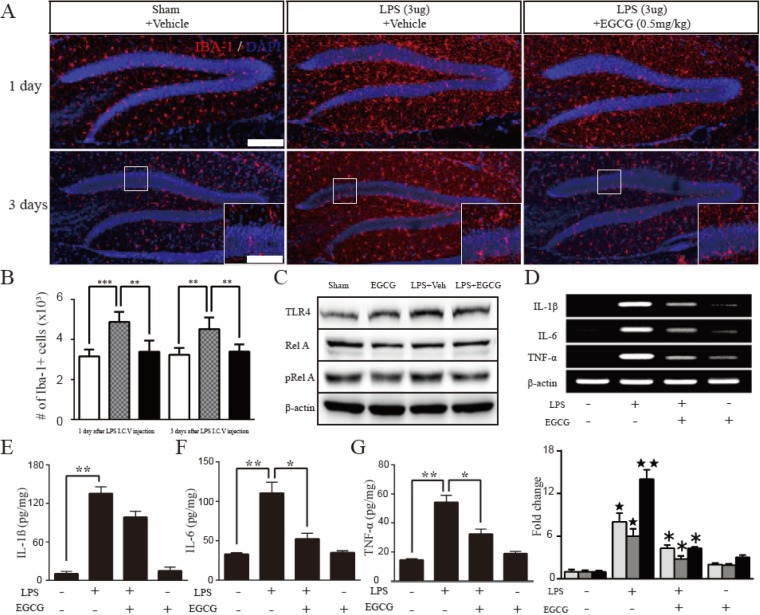
- Adult hippocampal dentate granule neurons are generated from neural stem cells (NSCs) in the mammalian brain, and the fate specification of adult NSCs is precisely controlled by the local niches and environment, such as the subventricular zone (SVZ), dentate gyrus (DG), and Toll-like receptors (TLRs). Epigallocatechin-3-gallate (EGCG) is the main polyphenolic flavonoid in green tea that has neuroprotective activities, but there is no clear understanding of the role of EGCG in adult neurogenesis in the DG after neuroinflammation. Here, we investigate the effect and the mechanism of EGCG on adult neurogenesis impaired by lipopolysaccharides (LPS). LPS-induced neuroinflammation inhibited adult neurogenesis by suppressing the proliferation and differentiation of neural stem cells in the DG, which was indicated by the decreased number of Bromodeoxyuridine (BrdU)-, Doublecortin (DCX)- and Neuronal Nuclei (NeuN)-positive cells. In addition, microglia were recruited with activatingTLR4-NF-kappaB signaling in the adult hippocampus by LPS injection. Treating LPS-injured mice with EGCG restored the proliferation and differentiation of NSCs in the DG, which were decreased by LPS, and EGCG treatment also ameliorated the apoptosis of NSCs. Moreover, pro-inflammatory cytokine production induced by LPS was attenuated by EGCG treatment through modulating the TLR4-NF-kappaB pathway. These results illustrate that EGCG has a beneficial effect on impaired adult neurogenesis caused by LPSinduced neuroinflammation, and it may be applicable as a therapeutic agent against neurodegenerative disorders caused by inflammation.
Keyword
MeSH Terms
Figure
Cited by 2 articles
-
Toll-like receptor 2 promotes neurogenesis from the dentate gyrus after photothrombotic cerebral ischemia in mice
Kyung-Joo Seong, Hyeong-Jun Kim, Bangrong Cai, Min-Suk Kook, Ji-Yeon Jung, Won-Jae Kim
Korean J Physiol Pharmacol. 2018;22(2):145-153. doi: 10.4196/kjpp.2018.22.2.145.Toll-like receptor 2 promotes neurogenesis from the dentate gyrus after photothrombotic cerebral ischemia in mice
Kyung-Joo Seong, Hyeong-Jun Kim, Bangrong Cai, Min-Suk Kook, Ji-Yeon Jung, Won-Jae Kim
Korean J Physiol Pharmacol. 2018;22(2):145-153. doi: 10.4196/kjpp.2018.22.2.145.
Reference
-
1. Ekdahl CT, Kokaia Z, Lindvall O. Brain inflammation and adult neurogenesis: the dual role of microglia. Neuroscience. 2009; 158:1021–1029. PMID: 18662748.
Article2. Polazzi E, Monti B. Microglia and neuroprotection: from in vitro studies to therapeutic applications. Prog Neurobiol. 2010; 92:293–315. PMID: 20609379.
Article3. Dheen ST, Kaur C, Ling EA. Microglial activation and its implications in the brain diseases. Curr Med Chem. 2007; 14:1189–1197. PMID: 17504139.4. Lourbopoulos A, Ertürk A, Hellal F. Microglia in action: how aging and injury can change the brain's guardians. Front Cell Neurosci. 2015; 9:54. PMID: 25755635.
Article5. Casano AM, Peri F. Microglia: multitasking specialists of the brain. Dev Cell. 2015; 32:469–477. PMID: 25710533.
Article6. Delpech JC, Madore C, Nadjar A, Joffre C, Wohleb ES, Layé S. Microglia in neuronal plasticity: Influence of stress. Neuropharmacology. 2015; 96:19–28. PMID: 25582288.
Article7. Bilimoria PM, Stevens B. Microglia function during brain development: New insights from animal models. Brain Res. 2015; 1617:7–17. PMID: 25463024.
Article8. Jeong JW, Lee HH, Han MH, Kim GY, Kim WJ, Choi YH. Anti-inflammatory effects of genistein via suppression of the toll-like receptor 4-mediated signaling pathway in lipopolysaccharide-stimulated BV2 microglia. Chem Biol Interact. 2014; 212:30–39. PMID: 24491678.
Article9. Su X, Chen Q, Chen W, Chen T, Li W, Li Y, Dou X, Zhang Y, Shen Y, Wu H, Yu C. Mycoepoxydiene inhibits activation of BV2 microglia stimulated by lipopolysaccharide through suppressing NF-κB, ERK 1/2 and toll-like receptor pathways. Int Immunopharmacol. 2014; 19:88–93. PMID: 24447679.
Article10. Park KW, Lee DY, Joe EH, Kim SU, Jin BK. Neuroprotective role of microglia expressing interleukin-4. J Neurosci Res. 2005; 81:397–402. PMID: 15948189.
Article11. Zhang Q, Yuan L, Liu D, Wang J, Wang S, Zhang Q, Gong Y, Liu H, Hao A, Wang Z. Hydrogen sulfide attenuates hypoxia-induced neurotoxicity through inhibiting microglial activation. Pharmacol Res. 2014; 84:32–44. PMID: 24788079.
Article12. Lehnardt S. Innate immunity and neuroinflammation in the CNS: the role of microglia in Toll-like receptor-mediated neuronal injury. Glia. 2010; 58:253–263. PMID: 19705460.
Article13. Matsuda T, Murao N, Katano Y, Juliandi B, Kohyama J, Akira S, Kawai T, Nakashima K. TLR9 signalling in microglia attenuates seizure-induced aberrant neurogenesis in the adult hippocampus. Nat Commun. 2015; 6:6514. PMID: 25751136.
Article14. Suhonen JO, Peterson DA, Ray J, Gage FH. Differentiation of adult hippocampus-derived progenitors into olfactory neurons in vivo. Nature. 1996; 383:624–627. PMID: 8857538.
Article16. Suh H, Deng W, Gage FH. Signaling in adult neurogenesis. Annu Rev Cell Dev Biol. 2009; 25:253–275. PMID: 19575663.
Article17. Wen S, Li H, Liu J. Dynamic signaling for neural stem cell fate determination. Cell Adh Migr. 2009; 3:107–117. PMID: 19262166.
Article18. Brown J, Cooper-Kuhn CM, Kempermann G, Van Praag H, Winkler J, Gage FH, Kuhn HG. Enriched environment and physical activity stimulate hippocampal but not olfactory bulb neurogenesis. Eur J Neurosci. 2003; 17:2042–2046. PMID: 12786970.
Article19. van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci. 1999; 2:266–270. PMID: 10195220.
Article20. Drapeau E, Mayo W, Aurousseau C, Le Moal M, Piazza PV, Abrous DN. Spatial memory performances of aged rats in the water maze predict levels of hippocampal neurogenesis. Proc Natl Acad Sci U S A. 2003; 100:14385–14390. PMID: 14614143.
Article21. Tatebayashi Y, Lee MH, Li L, Iqbal K, Grundke-Iqbal I. The dentate gyrus neurogenesis: a therapeutic target for Alzheimer's disease. Acta Neuropathol. 2003; 105:225–232. PMID: 12557008.
Article22. Abrous DN, Koehl M, Le Moal M. Adult neurogenesis: from precursors to network and physiology. Physiol Rev. 2005; 85:523–569. PMID: 15788705.
Article23. Rogers AE, Hafer LJ, Iskander YS, Yang S. Black tea and mammary gland carcinogenesis by 7,12-dimethylbenz[a]anthracene in rats fed control or high fat diets. Carcinogenesis. 1998; 19:1269–1273. PMID: 9683188.24. Zhou H, Chen JX, Yang CS, Yang MQ, Deng Y, Wang H. Gene regulation mediated by microRNAs in response to green tea polyphenol EGCG in mouse lung cancer. BMC Genomics. 2014; 15(Suppl 11):S3.
Article25. Singh BN, Shankar S, Srivastava RK. Green tea catechin, epigallocatechin-3-gallate (EGCG): mechanisms, perspectives and clinical applications. Biochem Pharmacol. 2011; 82:1807–1821. PMID: 21827739.
Article26. Lecumberri E, Dupertuis YM, Miralbell R, Pichard C. Green tea polyphenol epigallocatechin-3-gallate (EGCG) as adjuvant in cancer therapy. Clin Nutr. 2013; 32:894–903. PMID: 23582951.
Article27. Steinmann J, Buer J, Pietschmann T, Steinmann E. Anti-infective properties of epigallocatechin-3-gallate (EGCG), a component of green tea. Br J Pharmacol. 2013; 168:1059–1073. PMID: 23072320.
Article28. Gould E. How widespread is adult neurogenesis in mammals? Nat Rev Neurosci. 2007; 8:481–488. PMID: 17514200.
Article29. Lledo PM, Alonso M, Grubb MS. Adult neurogenesis and functional plasticity in neuronal circuits. Nat Rev Neurosci. 2006; 7:179–193. PMID: 16495940.
Article30. Kriegstein A, Alvarez-Buylla A. The glial nature of embryonic and adult neural stem cells. Annu Rev Neurosci. 2009; 32:149–184. PMID: 19555289.
Article31. Drew LJ, Fusi S, Hen R. Adult neurogenesis in the mammalian hippocampus: why the dentate gyrus? Learn Mem. 2013; 20:710–729. PMID: 24255101.
Article32. Singhal G, Jaehne EJ, Corrigan F, Toben C, Baune BT. Inflammasomes in neuroinflammation and changes in brain function: a focused review. Front Neurosci. 2014; 8:315. PMID: 25339862.
Article33. Monje ML, Toda H, Palmer TD. Inflammatory blockade restores adult hippocampal neurogenesis. Science. 2003; 302:1760–1765. PMID: 14615545.
Article34. Filiou MD, Arefin AS, Moscato P, Graeber MB. 'Neuroinflammation' differs categorically from inflammation: transcriptomes of Alzheimer's disease, Parkinson's disease, schizophrenia and inflammatory diseases compared. Neurogenetics. 2014; 15:201–212. PMID: 24928144.
Article35. Song C, Wang H. Cytokines mediated inf lammation and decreased neurogenesis in animal models of depression. Prog Neuropsychopharmacol Biol Psychiatry. 2011; 35:760–768. PMID: 20600462.36. Roy AM, Baliga MS, Katiyar SK. Epigallocatechin-3-gallate induces apoptosis in estrogen receptor-negative human breast carcinoma cells via modulation in protein expression of p53 and Bax and caspase-3 activation. Mol Cancer Ther. 2005; 4:81–90. PMID: 15657356.37. Abd El Mohsen MM, Kuhnle G, Rechner AR, Schroeter H, Rose S, Jenner P, Rice-Evans CA. Uptake and metabolism of epicatechin and its access to the brain after oral ingestion. Free Radic Biol Med. 2002; 33:1693–1702. PMID: 12488137.
Article38. Lin LC, Wang MN, Tseng TY, Sung JS, Tsai TH. Pharmacokinetics of (-)-epigallocatechin-3-gallate in conscious and freely moving rats and its brain regional distribution. J Agric Food Chem. 2007; 55:1517–1524. PMID: 17256961.
Article39. Li J, Ye L, Wang X, Liu J, Wang Y, Zhou Y, Ho W. (-)-Epigallocatechin gallate inhibits endotoxin-induced expression of inflammatory cytokines in human cerebral microvascular endothelial cells. J Neuroinflammation. 2012; 9:161. PMID: 22768975.
Article40. Mandel SA, Amit T, Weinreb O, Reznichenko L, Youdim MB. Simultaneous manipulation of multiple brain targets by green tea catechins: a potential neuroprotective strategy for Alzheimer and Parkinson diseases. CNS Neurosci Ther. 2008; 14:352–365. PMID: 19040558.
Article41. Avramovich-Tirosh Y, Reznichenko L, Mit T, Zheng H, Fridkin M, Weinreb O, Mandel S, Youdim MB. Neurorescue activity, APP regulation and amyloid-beta peptide reduction by novel multifunctional brain permeable iron-chelating-antioxidants, M-30 and green tea polyphenol, EGCG. Curr Alzheimer Res. 2007; 4:403–411. PMID: 17908043.42. Herges K, Millward JM, Hentschel N, Infante-Duarte C, Aktas O, Zipp F. Neuroprotective effect of combination therapy of glatiramer acetate and epigallocatechin-3-gallate in neuroinflammation. PLoS One. 2011; 6:e25456. PMID: 22022398.
Article43. Wu KJ, Hsieh MT, Wu CR, Wood WG, Chen YF. Green tea extract ameliorates learning and memory deficits in ischemic rats via its active component polyphenol epigallocatechin-3-gallate by modulation of oxidative stress and neuroinflammation. Evid Based Complement Alternat Med. 2012; 2012:163106. PMID: 22919410.
Article44. Weinreb O, Amit T, Youdim MB. A novel approach of proteomics and transcriptomics to study the mechanism of action of the antioxidant-iron chelator green tea polyphenol (-)-epigallocatechin-3-gallate. Free Radic Biol Med. 2007; 43:546–556. PMID: 17640565.
Article45. Weinreb O, Mandel S, Amit T, Youdim MB. Neurological mechanisms of green tea polyphenols in Alzheimer's and Parkinson's diseases. J Nutr Biochem. 2004; 15:506–516. PMID: 15350981.
Article46. Gan L, Meng ZJ, Xiong RB, Guo JQ, Lu XC, Zheng ZW, Deng YP, Luo BD, Zou F, Li H. Green tea polyphenol epigallocatechin-3-gallate ameliorates insulin resistance in non-alcoholic fatty liver disease mice. Acta Pharmacol Sin. 2015; 36:597–605. PMID: 25891086.
Article47. Wang Y, Li M, Xu X, Song M, Tao H, Bai Y. Green tea epigallocatechin-3-gallate (EGCG) promotes neural progenitor cell proliferation and sonic hedgehog pathway activation during adult hippocampal neurogenesis. Mol Nutr Food Res. 2012; 56:1292–1303. PMID: 22692966.
Article48. Yoo KY, Choi JH, Hwang IK, Lee CH, Lee SO, Han SM, Shin HC, Kang IJ, Won MH. (-)-Epigallocatechin-3-gallate increases cell proliferation and neuroblasts in the subgranular zone of the dentate gyrus in adult mice. Phytother Res. 2010; 24:1065–1070. PMID: 20013823.
Article49. Wang W, Deng M, Liu X, Ai W, Tang Q, Hu J. TLR4 activation induces nontolerant inf lammatory response in endothelial cells. Inflammation. 2011; 34:509–518. PMID: 20878353.50. Rolls A, Shechter R, London A, Ziv Y, Ronen A, Levy R, Schwartz M. Toll-like receptors modulate adult hippocampal neurogenesis. Nat Cell Biol. 2007; 9:1081–1088. PMID: 17704767.
Article51. Hong Byun E, Fujimura Y, Yamada K, Tachibana H. TLR4 signaling inhibitory pathway induced by green tea polyphenol epigallocatechin-3-gallate through 67-kDa laminin receptor. J Immunol. 2010; 185:33–45. PMID: 20511545.
Article52. Kumar P, Kumar A. Protective effects of epigallocatechin gallate following 3-nitropropionic acid-induced brain damage: possible nitric oxide mechanisms. Psychopharmacology (Berl). 2009; 207:257–270. PMID: 19763544.
Article53. Mao H, Fang X, Floyd KM, Polcz JE, Zhang P, Liu B. Induction of microglial reactive oxygen species production by the organochlorinated pesticide dieldrin. Brain Res. 2007; 1186:267–274. PMID: 17999924.
Article54. Levites Y, Amit T, Youdim MB, Mandel S. Involvement of protein kinase C activation and cell survival/cell cycle genes in green tea polyphenol (-)-epigallocatechin 3-gallate neuroprotective action. J Biol Chem. 2002; 277:30574–30580. PMID: 12058035.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Epigallocatechin-3-gallate Inhibits the Expression of Adhesion Molecules by Blocking Nuclear Factor Kappa B Signaling in Intestinal Epithelial Cells
- CKD-712, (S)-1-(alpha-naphthylmethyl)-6,7-dihydroxy-1,2,3,4-tetrahydroisoquinoline, Inhibits the NF-kappaB Activation and Augments Akt Activation during TLR4 Signaling
- Epigallocatechin-3-gallate Inhibits LPS-Induced NF-kappaB and MAPK Signaling Pathways in Bone Marrow-Derived Macrophages
- LPS Increases 5-LO Expression on Monocytes via an Activation of Akt-Sp1/NF-kappaB Pathways
- Paeoniflorin ameliorates neuropathic pain-induced depression-like behaviors in mice by inhibiting hippocampal neuroinflammation activated via TLR4/NF-kB pathway