Cancer Res Treat.
2015 Jul;47(3):501-508. 10.4143/crt.2014.054.
p21-Activated Kinase 4 (PAK4) as a Predictive Marker of Gemcitabine Sensitivity in Pancreatic Cancer Cell Lines
- Affiliations
-
- 1Division of Hematology and Medical Oncology, Department of Internal Medicine, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam, Korea. jwkim@snubh.org
- 2Department of Hematology and Medical Oncology, International St. Mary's Hospital, Incheon, Korea.
- KMID: 2148501
- DOI: http://doi.org/10.4143/crt.2014.054
Abstract
- PURPOSE
p21-activated kinases (PAKs) are involved in cytoskeletal reorganization, gene transcription, cell proliferation and survival, and oncogenic transformation. Therefore, we hypothesized that PAK expression levels could predict the sensitivity of pancreatic cancer cells to gemcitabine treatment, and PAKs could be therapeutic targets.
MATERIALS AND METHODS
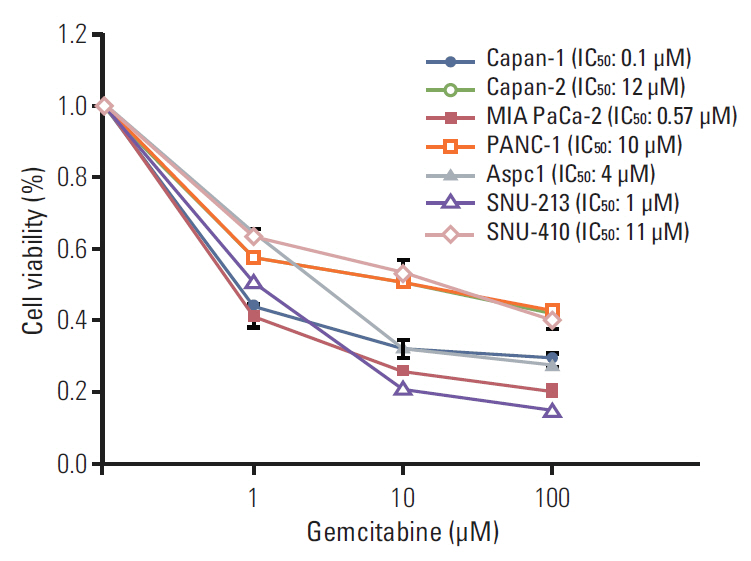
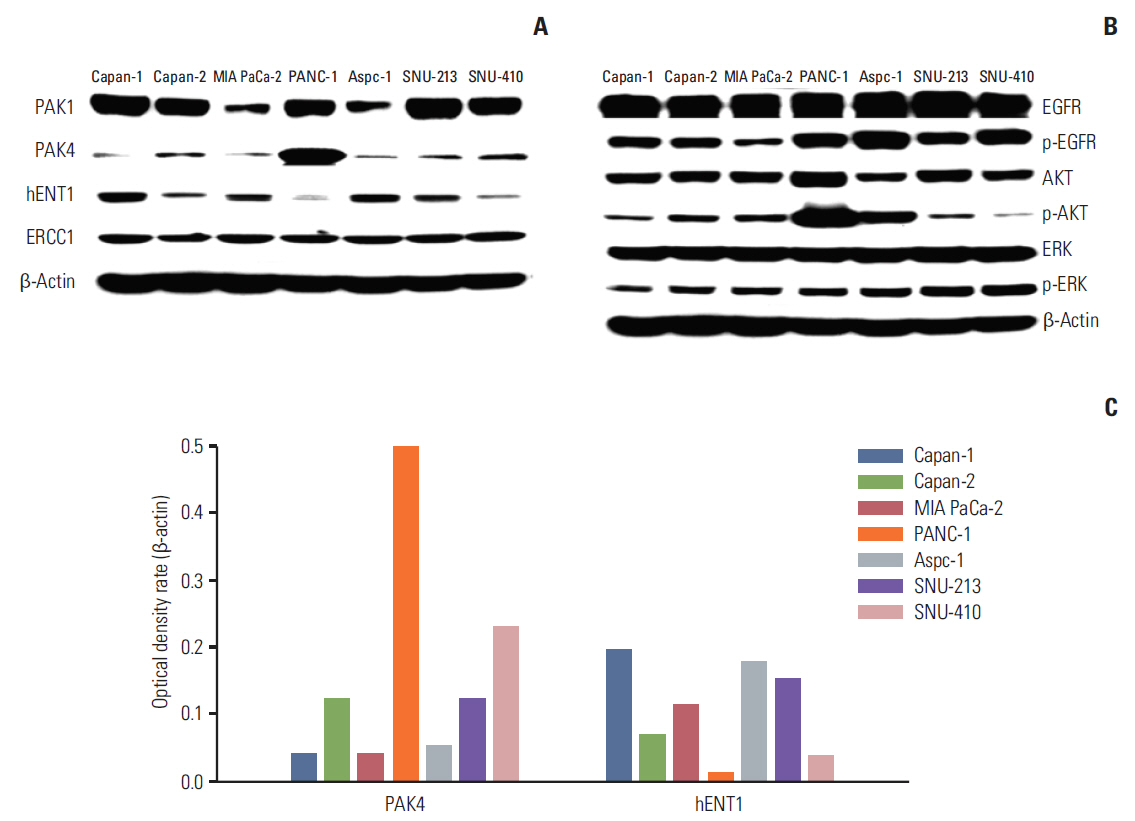
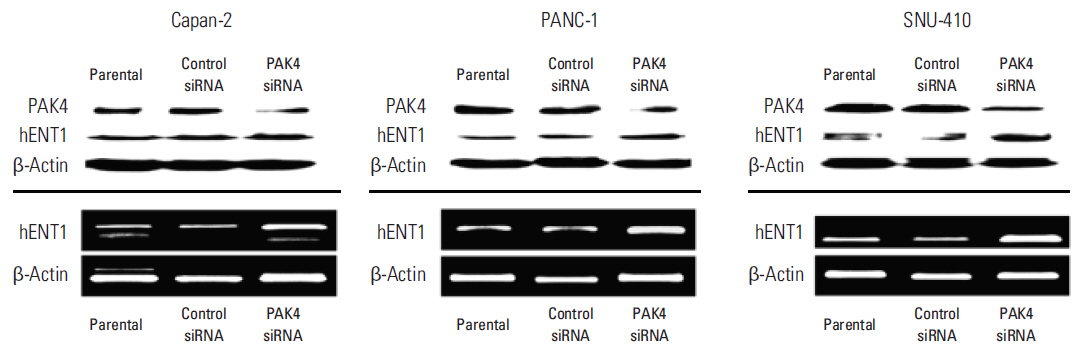
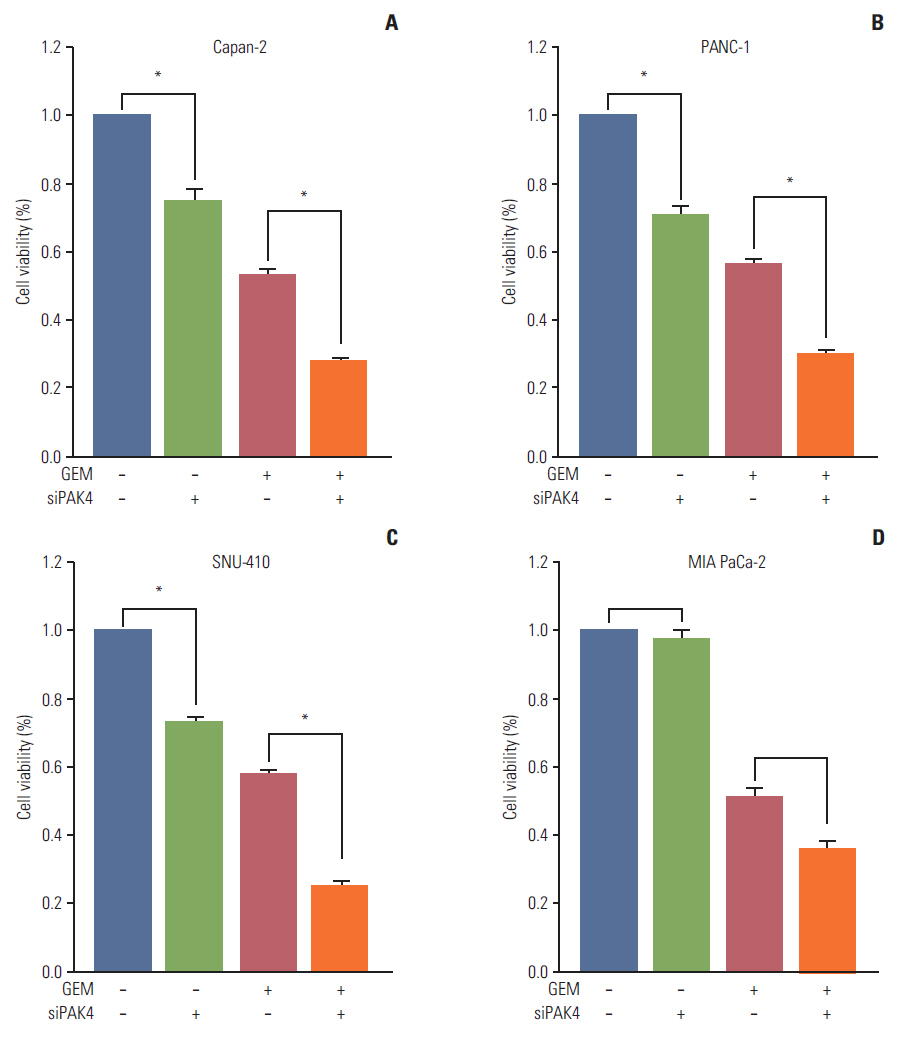
Cell viability inhibition by gemcitabine was evaluated in human pancreatic cancer cell lines (Capan-1, Capan-2, MIA PaCa-2, PANC-1, Aspc-1, SNU-213, and SNU-410). Protein expression and mRNA of molecules was detected by immunoblot analysis and reverse transcription polymerase chain reaction. To define the function of PAK4, PAK4 was controlled using PAK4 siRNA.
RESULTS
Capan-2, PANC-1, and SNU-410 cells were resistant to gemcitabine treatment. Immunoblot analysis of signaling molecules reported to indicate gemcitabine sensitivity showed higher expression of PAK4 and lower expression of human equilibrative nucleoside transporter 1 (hENT1), a well-known predictive marker for gemcitabine activity, in the resistant cell lines. Knockdown of PAK4 using siRNA induced the upregulation of hENT1. In resistant cell lines (Capan-2, PANC-1, and SNU-410), knockdown of PAK4 by siRNA resulted in restoration of sensitivity to gemcitabine.
CONCLUSION
PAK4 could be a predictive marker of gemcitabine sensitivity and a potential therapeutic target to increase gemcitabine sensitivity in pancreatic cancer.
Keyword
MeSH Terms
-
Cell Line*
Cell Proliferation
Cell Survival
Equilibrative Nucleoside Transporter 1
Humans
p21-Activated Kinases
Pancreatic Neoplasms*
Phosphotransferases*
Polymerase Chain Reaction
Reverse Transcription
RNA, Messenger
RNA, Small Interfering
Up-Regulation
Equilibrative Nucleoside Transporter 1
Phosphotransferases
RNA, Messenger
RNA, Small Interfering
p21-Activated Kinases
Figure
Reference
-
References
1. Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013; 369:1691–703.
Article2. Moore MJ, Goldstein D, Hamm J, Figer A, Hecht JR, Gallinger S, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007; 25:1960–6.
Article3. Colucci G, Labianca R, Di Costanzo F, Gebbia V, Carteni G, Massidda B, et al. Randomized phase III trial of gemcitabine plus cisplatin compared with single-agent gemcitabine as first-line treatment of patients with advanced pancreatic cancer: the GIP-1 study. J Clin Oncol. 2010; 28:1645–51.
Article4. Cunningham D, Chau I, Stocken DD, Valle JW, Smith D, Steward W, et al. Phase III randomized comparison of gemcitabine versus gemcitabine plus capecitabine in patients with advanced pancreatic cancer. J Clin Oncol. 2009; 27:5513–8.
Article5. Poplin E, Feng Y, Berlin J, Rothenberg ML, Hochster H, Mitchell E, et al. Phase III, randomized study of gemcitabine and oxaliplatin versus gemcitabine (fixed-dose rate infusion) compared with gemcitabine (30-minute infusion) in patients with pancreatic carcinoma E6201: a trial of the Eastern Cooperative Oncology Group. J Clin Oncol. 2009; 27:3778–85.
Article6. Oettle H, Post S, Neuhaus P, Gellert K, Langrehr J, Ridwelski K, et al. Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: a randomized controlled trial. JAMA. 2007; 297:267–77.7. Molli PR, Li DQ, Murray BW, Rayala SK, Kumar R. PAK signaling in oncogenesis. Oncogene. 2009; 28:2545–55.
Article8. Arias-Romero LE, Chernoff J. A tale of two Paks. Biol Cell. 2008; 100:97–108.
Article9. Dummler B, Ohshiro K, Kumar R, Field J. Pak protein kinases and their role in cancer. Cancer Metastasis Rev. 2009; 28:51–63.
Article10. Kumar R, Gururaj AE, Barnes CJ. p21-activated kinases in cancer. Nat Rev Cancer. 2006; 6:459–71.
Article11. Crawford JJ, Hoeflich KP, Rudolph J. p21-Activated kinase inhibitors: a patent review. Expert Opin Ther Pat. 2012; 22:293–310.
Article12. He H, Baldwin GS. p21-activated kinases and gastrointestinal cancer. Biochim Biophys Acta. 2013; 1833:33–9.
Article13. Ong CC, Jubb AM, Haverty PM, Zhou W, Tran V, Truong T, et al. Targeting p21-activated kinase 1 (PAK1) to induce apoptosis of tumor cells. Proc Natl Acad Sci U S A. 2011; 108:7177–82.
Article14. Chen S, Auletta T, Dovirak O, Hutter C, Kuntz K, El-ftesi S, et al. Copy number alterations in pancreatic cancer identify recurrent PAK4 amplification. Cancer Biol Ther. 2008; 7:1793–802.15. Lee SH, Jung YS, Chung JY, Oh AY, Lee SJ, Choi DH, et al. Novel tumor suppressive function of Smad4 in serum starvation-induced cell death through PAK1-PUMA pathway. Cell Death Dis. 2011; 2:e235.
Article16. Chauhan SC, Ebeling MC, Maher DM, Koch MD, Watanabe A, Aburatani H, et al. MUC13 mucin augments pancreatic tumorigenesis. Mol Cancer Ther. 2012; 11:24–33.
Article17. Deer EL, Gonzalez-Hernandez J, Coursen JD, Shea JE, Ngatia J, Scaife CL, et al. Phenotype and genotype of pancreatic cancer cell lines. Pancreas. 2010; 39:425–35.
Article18. Ku JL, Park JG. Biology of SNU cell lines. Cancer Res Treat. 2005; 37:1–19.
Article19. Qu J, Cammarano MS, Shi Q, Ha KC, de Lanerolle P, Minden A. Activated PAK4 regulates cell adhesion and anchorage-independent growth. Mol Cell Biol. 2001; 21:3523–33.
Article20. Li X, Minden A. PAK4 functions in tumor necrosis factor (TNF) alpha-induced survival pathways by facilitating TRADD binding to the TNF receptor. J Biol Chem. 2005; 280:41192–200.21. Siu MK, Yeung MC, Zhang H, Kong DS, Ho JW, Ngan HY, et al. p21-Activated kinase-1 promotes aggressive phenotype, cell proliferation, and invasion in gestational trophoblastic disease. Am J Pathol. 2010; 176:3015–22.
Article22. Ahn HK, Jang J, Lee J, Se Hoon P, Park JO, Park YS, et al. P21-activated kinase 4 overexpression in metastatic gastric cancer patients. Transl Oncol. 2011; 4:345–9.
Article23. Marechal R, Bachet JB, Mackey JR, Dalban C, Demetter P, Graham K, et al. Levels of gemcitabine transport and metabolism proteins predict survival times of patients treated with gemcitabine for pancreatic adenocarcinoma. Gastroenterology. 2012; 143:664–74.
Article24. Greenhalf W, Ghaneh P, Neoptolemos JP, Palmer DH, Cox TF, Lamb RF, et al. Pancreatic cancer hENT1 expression and survival from gemcitabine in patients from the ESPAC-3 trial. J Natl Cancer Inst. 2014; 106:djt347.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Expression of phosphorylated p21-activated kinase 4 is associated with aggressive histologic characteristics and poor prognosis in patients with surgically treated renal cell carcinoma
- α, γ-Mangostins Induce Autophagy and Show Synergistic Effect with Gemcitabine in Pancreatic Cancer Cell Lines
- p21-Activated Kinases (PAKs) as a Therapeutic Target
- Role of Mitogen Activated Protein Kinase and PI 3-kinase on Cell Proliferation in Pancreatic Cancer Cell Lines with K-ras Mutation
- Chemotherapy for Pancreatic Cancer