Korean J Urol.
2007 Feb;48(2):212-218. 10.4111/kju.2007.48.2.212.
Effects of Alpha-lipoic Acid on Nitric Oxide Synthase Expression and Ultrastructural Changes in the Bladder of Rats with Streptozotocin-induced Diabetes
- Affiliations
-
- 1Department of Urology, Inje University College of Medicine, Korea. kweonsikmin@ medimail.co.kr
- 2Paik Institute of Clinical Research, Korea.
- 3Department of Urology, Pusan National University College of Medicine, Busan, Korea.
- KMID: 2139763
- DOI: http://doi.org/10.4111/kju.2007.48.2.212
Abstract
-
PURPOSE: To evaluate whether alpha-lipoic acid (ALA) is effective at restoring the levels of nitric oxide synthase (NOS) expression and preventing ultrastructural changes in the bladder of rats with streptozotocin- induced diabetes.
MATERIALS AND METHODS
Nine-week-old male Sprague-Dawley rats were used. The experimental groups included a control group (n=6), a diabetes group (n=6), and two groups of diabetic rats treated with intraperitoneal injections of ALA (n=12) at either 50 (ALA50) or 100mg/kg/day (ALA100) for 8 weeks after the induction of diabetes. Diabetic oxidative stress was determined based on evaluation of immunohistochemical staining for 8-hydroxy-2-deoxyguanosine (8-OHdG). The measurements of the levels of eNOS and nNOS expressions, as well as an assessment of the ultrastructural changes in detrusor smooth muscle cells were performed.
RESULTS
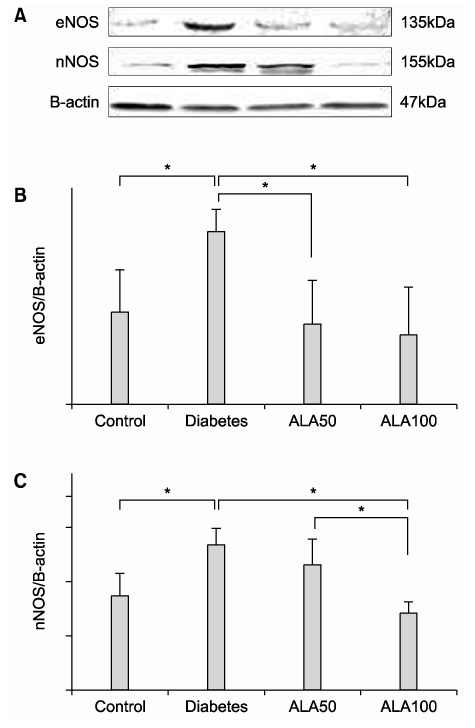
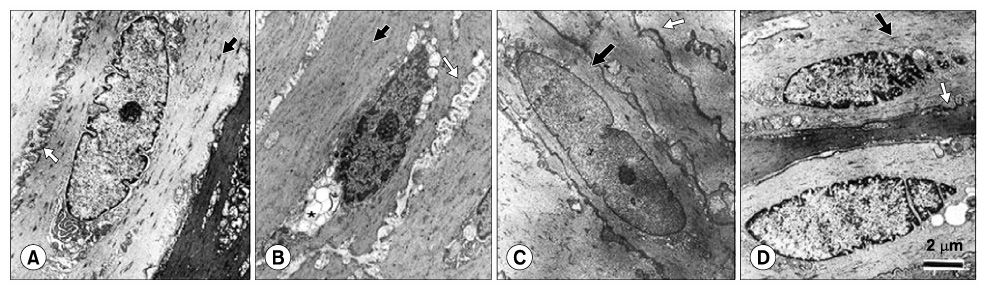
The highest expression of 8-OHdG was observed in the diabetes group; whereas, the 8-OHdG expression in the ALA-treated groups was similar to that in the control group. Both eNOS and nNOS were constitutively expressed in the control group. The expression levels of both eNOS and nNOS proteins were higher in the diabetes group, which had experienced increased oxidative stress, than in the ALA50 and ALA100 groups. Compared with the control group, the diabetes group exhibited severe degeneration of the detrusor muscle cells. In the rats treated with ALA, the detrusor muscle cells showed mild to moderate degeneration. The mean numbers of mitochondria per smooth muscle cell in the control, diabetes, ALA50 and ALA100 groups were 12.6+/-1.5, 5.1+/-0.7, 18.3+/-0.7 and 19.3+/-1.3, respectively (p<0.01). CONCLISIONS: Our data suggest that diabetes enhanced the levels of eNOS and nNOS expressions in the bladder, and ALA inhibited the expressions of eNOS and nNOS. ALA had a protective effect against the degeneration of intracellular micro-organelles produced by diabetic oxidative damage in detrusor muscle cells. This study suggests that early treatment with ALA can reduce the damage caused by diabetic oxidative stress.
Keyword
MeSH Terms
Figure
Reference
-
1. Kim SA, Park WS, Ohrr HC, Kang HY, Lee DH, Yi SW, et al. Prevalence and management status of diabetes mellitus in Korea. Korean J Med. 2005. 68:10–17.2. Brown JS, Wessells H, Chancellor MB, Howards SS, Stamm WE, Stapleton AE, et al. Urologic complications of diabetes. Diabetes Care. 2005. 28:177–185.3. Wolff SP. Diabetes mellitus and free radicals. Free radicals, transition metals and oxidative stress in the aetiology of diabetes mellitus and complications. Br Med Bull. 1993. 49:642–645.4. Sies H, Stahl W. Vitamins E and C, beta-carotene, and other carotenoids as antioxidants. Am J Clin Nutr. 1995. 62(6):Suppl. 1315S–1321S.5. Reed LJ, DeBusk BG, Gunsalus IC, Hornberger CS Jr. Crystalline alpha-lipoic acid: a catalytic agent associated with pyruvate dehydrogenase. Science. 1951. 27:93–94.6. Packer L, Witt EH, Tritsckler HJ. Alpha-lipoic acid as a biological antioxidant. Free Radic Biol Med. 1995. 19:227–250.7. Berman JR, McCarthy MM, Kyprianou N. Effect of estrogen withdrawal on nitric oxide synthase expression and apoptosis in the rat vagina. Urology. 1998. 51:650–656.8. Khamaisi M, Potashnik R, Tirosh A, Demshchak E, Rudich A, Tritschler H, et al. Lipoic acid reduces glycemia and increases muscle GLUT4 content in streptozotocin-diabetic rats. Metabolism. 1997. 46:763–768.9. Maritim AC, Sanders RA, Watkins JB 3rd. Effects of alpha-lipoic acid on biomarkers of oxidative stress in streptozotocin-induced diabetic rats. J Nutr Biochem. 2003. 14:288–294.10. Arivazhagan P, Ramanathan K, Panneerselvam C. Effect of DL-alpha-lipoic acid on mitochondrial enzymes in aged rats. Chem Biol Interact. 2001. 138:189–198.11. Jacob S, Streeper RS, Fogt DL, Hokama JY, Tritschler HJ, Dietze GJ, et al. The antioxidant alpha-lipoic acid enhances insulin-stimulated glucose metabolism in insulin-resistant rat skeletal muscle. Diabetes. 1996. 45:1024–1029.12. Khamaisi M, Rudich A, Potashnik R, Tritschler HJ, Gutman A, Bashan N. Lipoic acid acutely induces hypoglycemia in fasting nondiabetic and diabetic rats. Metabolism. 1999. 48:504–510.13. Leinonen J, Lehtimaki T, Toyokuni S, Okada K, Tanaka T, Hiai H, et al. New biomarker evidence of oxidative DNA damage in patients with non-insulin-dependent diabetes mellitus. FEBS Lett. 1997. 417:150–152.14. Ihara Y, Toyokuni S, Uchida K, Odaka H, Tanaka T, Ikeda H, et al. Hyperglycemia causes oxidative stress in pancreatic beta-cells of GK rats, a model of type 2 diabetes. Diabetes. 1999. 48:927–932.15. Cosentino F, Hishikawa K, Katusic ZS, Luscher TF. High glucose increases nitric oxide synthase expression and superoxide anion generation in human aortic endothelial cells. Circulation. 1997. 96:25–28.16. Sen CK, Roy S, Han D, Packer L. Regulation of cellular thiols in human lymphocytes by alpha-lipoic acid: a flow cytometric analysis. Free Radic Biol Med. 1997. 22:1241–1257.17. Heitzer T, Krohn K, Albers S, Meinertz T. Tetrahydrobiopterin improves endothelium-dependent vasodilation by increasing nitric oxide activity in patients with Type II diabetes mellitus. Diabetologia. 2000. 43:1435–1438.18. Minamiyama Y, Bito Y, Takemura S, Takahashi Y, Kodai S, Mizuguchi S, et al. Calorie restriction improves cardiovascular risk factors via reduction of mitochondrial reactive oxygen species in type II diabetic rats. J Pharmacol Exp Ther. 2007. 320:535–543.19. Jesmin S, Zaedi S, Maeda S, Yamaguchi I, Goto K, Miyauchi T. Effects of a selective endothelin a receptor antagonist on the expressions of iNOS and eNOS in the heart of early streptozotocin-induced diabetic rats. Exp Biol Med (Maywood). 2006. 231:925–931.20. Park HJ, Park BK, Park NC. Penile expression of hypoxiainducible factor-1alpha, vascular endothelial growth factor, and nitric oxide synthase in the type II diabetic rat. Korean J Androl. 2005. 23:144–152.21. Oh BR, Kim HJ, Ahn KY, Kwon DD, Ryu SB, Park YI. Change of nitric oxide synthase gene and protein expressions after bacillus calmette-guerin instillation into rat bladder. Korean J Urol. 2001. 42:413–419.22. Surendran S, Kondapaka SB. Altered expression of neuronal nitric oxide synthase in the duodenum longitudinal musclemyenteric plexus of obesity induced diabetes mouse: implications on enteric neurodegeneration. Biochem Biophys Res Commun. 2005. 338:919–922.23. Shotton HR, Clarke S, Lincoln J. The effectiveness of treatments of diabetic autonomic neuropathy is not the same in autonomic nerves supplying different organs. Diabetes. 2003. 52:157–164.24. Park JW, Park SJ, Park SH, Kim KY, Chung JW, Chun MH, et al. Up-regulated expression of neuronal nitric oxide synthase in experimental diabetic retina. Neurobiol Dis. 2006. 21:43–49.25. Elbadawi A. Functional pathology of urinary bladder muscularis: the new frontier in diagnostic uropathology. Semin Diagn Pathol. 1993. 10:314–354.26. Nishikawa T, Edelstein D, Du XL, Yamagishi S, Matsumura T, Kaneda Y, et al. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature. 2000. 404:787–790.27. Skulachev VP. Bioenergetic aspects of apoptosis, necrosis and mitoptosis. Apoptosis. 2006. 11:473–485.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Expression of NADPH-diaphorase in Corneal Neovascularization of streptozotocin Induced Diabetic Rat
- Impaired endothelium-dependent relaxation is mediated by reduced production of nitric oxide in the streptozotocin-induced diabetic rats
- Involvement of Fibronectin in the Migration of Macrophage and Expression of Nitric Oxide Synthase in the BCG induced Inflammatory Sites in Rat Bladder
- Inhibitory Effect of Esculetin on the Inducuble Nitric Oxide Synthase Expression in TNF-stimulated 3T3-L1 Adipocytes
- Teat Shock Response Ingibits IFN-gamma Plus LPS - Induced NO Synthase Expression in Murine Peritoneal Macrophages