Nutr Res Pract.
2008 Dec;2(4):317-321. 10.4162/nrp.2008.2.4.317.
Effects of various metal ions on the gene expression of iron exporter ferroportin-1 in J774 macrophages
- Affiliations
-
- 1Department of Food & Nutrition, Kyung Hee University, 1 Hoegi-dong, Dongdaemun-gu, Seoul 130-701, Korea. jchung@khu.ac.kr
- KMID: 2139209
- DOI: http://doi.org/10.4162/nrp.2008.2.4.317
Abstract
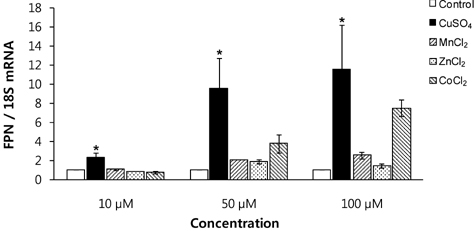
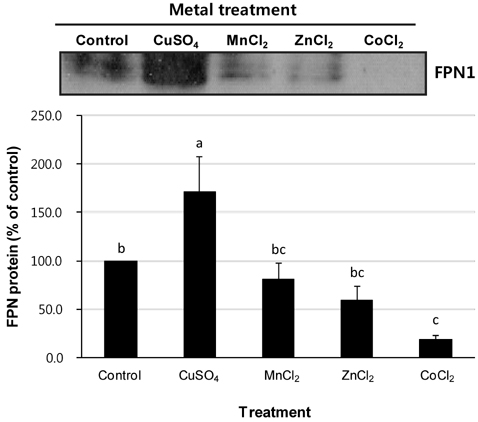
- Macrophages play a key role in iron metabolism by recycling iron through erythrophagocytosis. Ferroportin-1 (FPN1) is a transporter protein that is known to mediate iron export from macrophages. Since divalent metals often interact with iron metabolism, we examined if divalent metals could regulate the expression of FPN1 in macrophages. J774 macrophage cells were treated with copper, manganese, zinc, or cobalt at 10, 50, or 100 microM for 16 to 24 h. Then, FPN1 mRNA and protein levels were determined by quantitative real-time PCR and Western blot analyses, respectively. In addition, effects of divalent metals on FPN1 promoter activity were examined by luciferase reporter assays. Results showed that copper significantly increased FPN1 mRNA levels in a dose-dependent manner. The copper-induced expression of FPN1 mRNA was associated with a corresponding increase in FPN1 protein levels. Also, copper directly stimulated the activity of FPN1 promoter-driven reporter construct. In contrast, manganese and zinc had no effect on the FPN1 gene expression in J774 cells. Interestingly, cobalt treatment in J774 cells decreased FPN1 protein levels without affecting FPN1 mRNA levels. In conclusion, our study results demonstrate that divalent metals differentially regulate FPN1 expression in macrophages and indicate a potential interaction of divalent metals with the FPN1-mediated iron export in macrophages.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Cadmium increases ferroportin-1 gene expression in J774 macrophage cells via the production of reactive oxygen species
Bo-yeon Park, Jayong Chung
Nutr Res Pract. 2009;3(3):192-199. doi: 10.4162/nrp.2009.3.3.192.
Reference
-
1. Abboud S, Haile DJ. A novel mammalian iron-regulated protein involved in intracellular iron metabolism. J Biol Chem. 2000. 275:19906–19912.
Article2. Aigner E, Theurl I, Haufe H, Seifert M, Hohla F, Scharinger L, Stickel F, Mourlane F, Weiss G, Datz C. Copper availability contributes to iron perturbations in human nonalcoholic fatty liver disease. Gastroenterology. 2008. 135:680–688.
Article3. Andersen HS, Gambling L, Holtrop G, McArdle HJ. Effect of dietary copper deficiency on iron metabolism in the pregnant rat. Br J Nutr. 2007. 97:239–246.
Article4. Beutler E. Hemochromatosis: genetics and pathophysiology. Annu Rev Med. 2006. 57:331–347.
Article5. Chung J, Haile DJ, Wessling-Resnick M. Copper-induced ferroportin-1 expression in J774 macrophages is associated with increased iron efflux. Proc Natl Acad Sci U S A. 2004. 101:2700–2705.
Article6. Cuthbert JA. Wilson's disease: a new gene and an animal model for an old disease. J Investig Med. 1995. 43:323–336.7. Donovan A, Brownlie A, Zhou Y, Shepard J, Pratt SJ, Moynihan J, Paw BH, Drejer A, Barut B, Zapata A, Law TC, Brugnara C, Lux SE, Pinkus GS, Pinkus JL, Kingsley PD, Palis J, Fleming MD, Andrews NC, Zon LI. Positional cloning of zebrafish ferroportin1 identifies a conserved vertebrate iron exporter. Nature. 2000. 403:776–781.
Article8. Ganz T, Nemeth E. Regulation of iron acquisition and iron distribution in mammals. Biochim Biophys Acta. 2006. 1763:690–699.
Article9. Giedroc DP, Chen X, Apuy JL. Metal response element (MRE)-binding transcription factor-1 (MTF-1): structure, function, and regulation. Antioxid Redox Signal. 2001. 3:577–596.
Article10. González M, Reyes-Jara A, Suazo M, Jo WJ, Vulpe C. Expression of copper-related genes in response to copper load. Am J Clin Nutr. 2008. 88:830S–834S.
Article11. Gunshin H, Mackenzie B, Berger UV, Gunshin Y, Romero MF, Boron WF, Nussberger S, Gollan JL, Hediger MA. Cloning and characterization of a mammalian proton-coupled metal-ion transporter. Nature. 1997. 388:482–488.
Article12. Hunt JR, Matthys LA, Johnson LK. Zinc absorption, mineral balance, and blood lipids in women consuming controlled lactoovovegetarian and omnivorous diets for 8 wk. Am J Clin Nutr. 1998. 67:421–430.
Article13. Itoh S, Kim HW, Nakagawa O, Ozumi K, Lessner SM, Aoki H, Akram K, McKinney RD, Ushio-Fukai M, Fukai T. Novel role of antioxidant-1 (Atox1) as a copper-dependent transcription factor involved in cell proliferation. J Biol Chem. 2008. 283:9157–9167.
Article14. Knutson MD, Oukka M, Koss LM, Aydemir F, Wessling-Resnick M. Iron release from macrophages after erythrophagocytosis is up-regulated by ferroportin 1 overexpression and down-regulated by hepcidin. Proc Natl Acad Sci U S A. 2005. 102:1324–1328.
Article15. Knutson MD, Vafa MR, Haile DJ, Wessling-Resnick M. Iron loading and erythrophagocytosis increase ferroportin 1 (FPN1) expression in J774 macrophages. Blood. 2003. 102:4191–4197.
Article16. Li GJ, Zhang LL, Lu L, Wu P, Zheng W. Occupational exposure to welding fume among welders: alterations of manganese, iron, zinc, copper, and lead in body fluids and the oxidative stress status. J Occup Environ Med. 2004. 46:241–248.
Article17. Liu XB, Hill P, Haile DJ. Role of the ferroportin iron-responsive element in iron and nitric oxide dependent gene regulation. Blood Cells Mol Dis. 2002. 29:315–326.
Article18. Ludwiczek S, Aigner E, Theurl I, Weiss G. Cytokine-mediated regulation of iron transport in human monocytic cells. Blood. 2003. 101:4148–4154.
Article19. Mattie MD, Freedman JH. Copper-inducible transcription: regulation by metal- and oxidative stress-responsive pathways. Am J Physiol Cell Physiol. 2004. 286:C293–C301.
Article20. McKie AT, Marciani P, Rolfs A, Brennan K, Wehr K, Barrow D, Miret S, Bomford A, Peters TJ, Farzaneh F, Hediger MA, Hentze MW, Simpson RJ. A novel duodenal iron-regulated transporter, IREG1, implicated in the basolateral transfer of iron to the circulation. Mol Cell. 2000. 5:299–309.
Article21. Muller PA, Klomp LW. ATOX1: A novel copper-responsive transcription factor in mammals? Int J Biochem Cell Biol. 2008. BC-2807:1–4.
Article22. Muller PA, van Bakel H, van de Sluis B, Holstege F, Wijmenga C, Klomp LW. Gene expression profiling of liver cells after copper overload in vivo and in vitro reveals new copper-regulated genes. J Biol Inorg Chem. 2007. 12:495–507.
Article23. Pietrangelo A. The ferroportin disease. Blood Cells Mol Dis. 2004. 32:131–138.
Article24. Song IS, Chen HH, Aiba I, Hossain A, Liang ZD, Klomp LW, Kuo MT. Transcription factor Sp1 plays an important role in the regulation of copper homeostasis in mammalian cells. Mol Pharmacol. 2008. 74:705–713.
Article25. Wang X, Miller DS, Zheng W. Intracellular localization and subsequent redistribution of metal transporters in a rat choroid plexus model following exposure to manganese or iron. Toxicol Appl Pharmacol. 2008. 230:167–174.
Article26. Weiss G. Iron and immunity: a double-edged sword. Eur J Clin Invest. 2002. 32:70–78.
Article27. Yamaji S, Tennant J, Tandy S, Williams M, Singh Srai SK, Sharp P. Zinc regulates the function and expression of the iron transporters DMT1 and IREG1 in human intestinal Caco-2 cells. FEBS Lett. 2001. 507:137–141.
Article28. Yang F, Liu XB, Quinones M, Melby PC, Ghio A, Haile DJ. Regulation of reticuloendothelial iron transporter MTP1 (Slc11a3) by inflammation. J Biol Chem. 2002b. 277:39786–39791.
Article29. Yang F, Wang X, Haile DJ, Piantadosi CA, Ghio AJ. Iron increases expression of iron-export protein MTP1 in lung cells. Am J Physiol Lung Cell Mol Physiol. 2002a. 283:L932–L939.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of Copper on the Regulation of Ferroportin-1 Gene Expression
- Cadmium increases ferroportin-1 gene expression in J774 macrophage cells via the production of reactive oxygen species
- Metal-on-metal Articulation of Total Hip Replacement
- Chronic Opisthorchis viverrini Infection and Associated Hepatobiliary Disease Is Associated with Iron Loaded M2-like Macrophages
- Metal-on-Metal Bearing in Total Hip Arthroplasty