Nutr Res Pract.
2008 Dec;2(4):195-199. 10.4162/nrp.2008.2.4.195.
In vitro inhibition of 10-formyltetrahydrofolate dehydrogenase activity by acetaldehyde
- Affiliations
-
- 1Department of Food and Nutrition, College of Life Science and Nano Technology, Hannam University, 461-6 Jeonmin-dong, Yuseong-gu, Daejeon 305-811, Korea. hsmin@hnu.kr
- 2Department of Biological Sciences, Korea Advanced Institute of Science and Technology, 373-1 Guseong-dong, Yuseong-gu, Daejeon 305-701, Korea.
- KMID: 2139191
- DOI: http://doi.org/10.4162/nrp.2008.2.4.195
Abstract
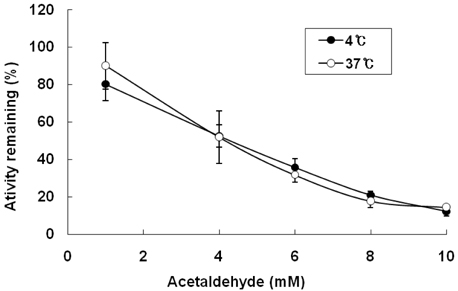
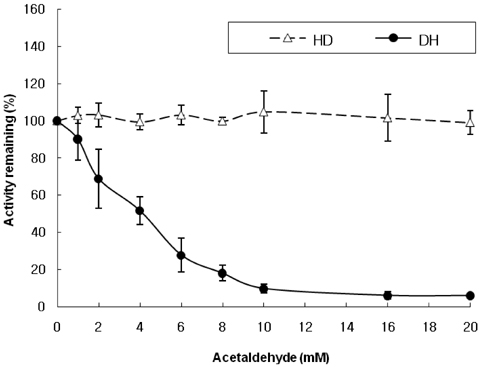
- Alcoholism has been associated with folate deficiency in humans and laboratory animals. Previous study showed that ethanol feeding reduces the dehydrogenase and hydrolase activity of 10-formyltetrahydrofolate dehydrogenase (FDH) in rat liver. Hepatic ethanol metabolism generates acetaldehyde and acetate. The mechanisms by which ethanol and its metabolites produce toxicity within the liver cells are unknown. We purified FDH from rat liver and investigated the effect of ethanol, acetaldehyde and acetate on the enzyme in vitro. Hepatic FDH activity was not reduced by ethanol or acetate directly. However, acetaldehyde was observed to reduce the dehydrogenase activity of FDH in a dose- and time-dependent manner with an apparent IC50 of 4 mM, while the hydrolase activity of FDH was not affected by acetaldehyde in vitro. These results suggest that the inhibition of hepatic FDH dehydrogenase activity induced by acetadehyde may play a role in ethanol toxicity.
MeSH Terms
Figure
Reference
-
1. Agarwal DP. Genetic polymorphisms of alcohol metabolizing enzymes. Pathol Biol. 2001. 49:703–709.
Article2. Barak AJ, Beckenhauer HC, Tuma DJ. Methionine synthase, a possible prime site of the ethanolic lesion in liver. Alcohol. 2001. 26:65–67.3. Barak AJ, Beckenhauer HC, Malliard ME, Kharbanda KK, Tuma DJ. Betaine lowers elevated s-adenosylhomocysteine levels in hepatocytes from ethanol-fed rats. J Nutr. 2003. 133:2845–2848.
Article4. Blasiak J, Trzeciak A, Malecka-Panas E, Drzewoski J, Wojewódzka M. In vitro genotoxicity of ethanol and acetaldehyde in human lymphocytes and the gastrointestinal tract mucosa cells. Toxicol In Vitro. 2000. 14:287–295.
Article5. Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976. 72:248–254.
Article6. Chen J, Petersen DR, Schenker S, Henderson GI. Formation of malondialdehyde adducts in livers of rats exposed to ethanol: role in ethanol-mediated inhibition of cytochrome c oxidase. Alcohol Clin Exp Res. 2000. 24:544–552.
Article7. Cook RJ, Lloyd RS, Wagner C. Isolation and characterization of cDNA clones for rat liver 10-formyltetrahydrofolate dehydrogenase. J Biol Chem. 1991. 266:4965–4973.
Article8. Cook RJ, Wagner C. Enzymatic activities of rat liver cytosol 10-formyltetrahydrofolate dehydrogenase. Arch Biochem Biophys. 1995. 321:336–344.
Article9. Diehl AM. Liver disease in alcohol abusers: clinical perspective. Alcohol. 2002. 27:7–11.10. Donato H, Krupenko NI, Tsybovsky Y, Krupenko SA. 10-formyltetrahydrofolate dehydrogenase requires 4'-phosphopantetheine prosthetic froup for catalysis. J Biol Chem. 2008. 282:34159–34166.
Article11. Donohue TM, Tuma DJ, Sorrell MF. Acetaldehyde adducts with proteins: binding of [14C] acetaldehyde to serum albumin. Arch Biochem Biophys. 1983. 220:239–246.
Article12. Eriksson CJ, Sippel HW. The distribution and metabolism of acetaldehyde in rats during ethanol oxidation-I. The distribution of acetaldehyde in liver, brain, blood and breath. Biochem Pharmacol. 1977. 26:241–247.
Article13. Hard ML, Raha S, Spino M, Robinson BH, Koren G. Impairment of pyruvate dehydrogenase activity by acetaldehyde. Alcohol. 2001. 25:1–8.
Article14. Horne DW, Briggs WT, Wagner C. Ethanol stimulates 5-methyltetrahydrofolate accumulation in isolated rat liver cells. Biochem Pharmacol. 1978. 27:2069–2074.
Article15. Im ES, Seo J, Min H. Effects of chronic ethanol administration on folate metabolism and plasma homocysteine concentration in the rats. The Korean Journal of Nutrition. 1998. 31:1006–1013.16. Israel Y, Orrego H, Carmichael FJ. Acetate-mediated effects of ethanol. Alcohol Clin Exp Res. 1994. 18:144–148.
Article17. Kenyon SH, Nicolaou A, Gibbons WA. The effect of ethanol and its metabolites upon methionine synthase activity in vitro. Alcohol. 1998. 15:305–309.
Article18. Kim S, Park GH, Joo WA, Paik WK, Cook RJ, Williams KR. Identification of protein-arginine N-methyltransferase as 10-formyltetrahydrofolate dehydrogenase. J Biol Chem. 1998. 273:27374–27382.
Article19. Krupenko SA, Wagner C, Cook RJ. Cysteine 707 is involved in the dehydrogenase activity site of rat 10-formyltetrahydrofolate dehydrogenase. J Biol Chem. 1995. 270:519–522.
Article20. Krupenko SA, Wagner C, Cook RJ. Domain structure of rat 10-formyltetrahydrofolate dehydrogenase. Resolution of the amino-terminal domain as 10-formyltetrahydrofolate hydrolase. J Biol Chem. 1997. 272:10273–10278.21. Kutzbach C, Stokstad EL. Mammalian methylenetetrahydrofolate reductase. Partial purification, properties, and inhibition by S-adenosylmethionine. Biochim Biophys Acta. 1971. 250:459–477.
Article22. Lieber CS. Alcoholism: a disease of internal medicine. J Stud Alcohol. 1990. 51:101–103.
Article23. Min H, Im ES, Seo JS, Mun JA, Burri BJ. Effects of chronic ethanol ingestion and folate deficiency on the activity of 10-formyltetrahydrofolate dehydrogenase in rat liver. Alcohol Clin Exp Res. 2005. 29:2188–2193.
Article24. Min H, Shane B, Stokstad EL. Identification of 10-formytetrahydrofolate dehydrofenase as a cytosolic folate binding protein in rat liver. Biochim Biophys Acta. 1988. 967:348–353.
Article25. Neuman MG, Katz GG, Malkiewicz IM, Mathurin P, Tsukamoto H, Adachi M, Ishii H, Colell A, Garcia-Ruiz C, Fernandez-Checa JC, Casey CA. Alcoholic liver injury and apoptosis-synopsis of the symposium held at ESBRA 2001: 8th Congress of the European Society for Biomedical Research on Alcoholism, Paris, September 16, 2006. Alcohol. 2001. 28:117–128.26. Nicholls RM, Fowles LF, Worrall S, de Jersey J, Wilce PA. Distribution and turnover of acetaldehyde-modified proteins in liver and blood of ethanol-fed rats. Alcohol Alcohol. 1994. 29:149–157.27. Oneta CM, Lieber CS, Li J, Ruttimann S, Schmid B, Lattmann J, Rosman AS, Seitz HK. Dynamics of cytochrome P4502E1 activity in man: induction by ethanol and disappearance during withdrawal phase. J Hepatol. 2002. 36:47–52.
Article28. Pumford NR, Halmes NC, Martin BM, Cook RJ, Wagner C, Hinson JA. Covalent binding of acetaminophen to N-10-formyltetrahydrofolate dehydrogenase in mice. J Pharmacol Exp Ther. 1997. 280:501–505.29. Rios-Orlandi EM, Zarkadas CG, MacKenzie RE. Formyltetrahydrofolate dehydrogenase-hydrolase from pig liver: simultaneous assay of the activities. Biochim Biophys Acta. 1986. 87:24–35.
Article30. Rouach H, Andraud E, Aufrère G, Beaugé F. The effects of acetaldehyde in vitro on proteasome activities and its potential involvement after alcoholization of rats by inhalation of ethanol vapours. Alcohol Alcohol. 2005. 40:359–366.
Article31. Schirch D, Villar E, Maras B, Barra D, Schirch V. Domain structure and function of 10-formyltetrahydrofolate dehydrogenase. J Biol Chem. 1994. 269:24728–24735.
Article32. Scrutton MC, Beis I. Inhibitory effects of histidine and their reversal. The roles of pyruvate carboxylase and N10-formyltetrahydrofolate dehydrogenase. Biochem J. 1979. 177:833–846.
Article33. Tamura T, Halsted CH. Folate turnover in chronically alcoholic monkeys. J Lab Clin Med. 1983. 101:623–628.34. Worrall S, Thiele GM. Protein modification in ethanol toxicity. Adverse Drug React Toxicol Rev. 2001. 20:133–159.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Epileptic Seizure Due to Disulfiram Treatment
- Effects of acetaldehyde on responses of rabbit corpus cavernosal smooth muscle
- Effect of the Mixture of Pueraria lobata and Sorbus commixta Extract on the Alcohol-induced Hangover in Rats
- The influence of H1, H2-histamine antagonists and disulfiram to ethanol and acetaldehyde patch test results
- Hypotension Caused by a Disulfiram-Alcohol Reaction