J Korean Assoc Oral Maxillofac Surg.
2012 Dec;38(6):343-353. 10.5125/jkaoms.2012.38.6.343.
Isolation of human mesenchymal stem cells from the skin and their neurogenic differentiation in vitro
- Affiliations
-
- 1Department of Oral and Maxillofacial Surgery, School of Medicine and Institute of Health Science, Gyeongsang National University, Jinju, Korea. parkbw@gnu.ac.kr
- 2OBS/Theriogenology and Biotechnology, College of Veterinary Medicine, Gyeongsang National University, Jinju, Korea.
- 3Department of Neurosurgery, School of Medicine, Gyeongsang National University, Jinju, Korea.
- KMID: 2136971
- DOI: http://doi.org/10.5125/jkaoms.2012.38.6.343
Abstract
OBJECTIVES
This aim of this study was to effectively isolate mesenchymal stem cells (hSMSCs) from human submandibular skin tissues (termed hSMSCs) and evaluate their characteristics. These hSMSCs were then chemically induced to the neuronal lineage and analyzed for their neurogenic characteristics in vitro.
MATERIALS AND METHODS
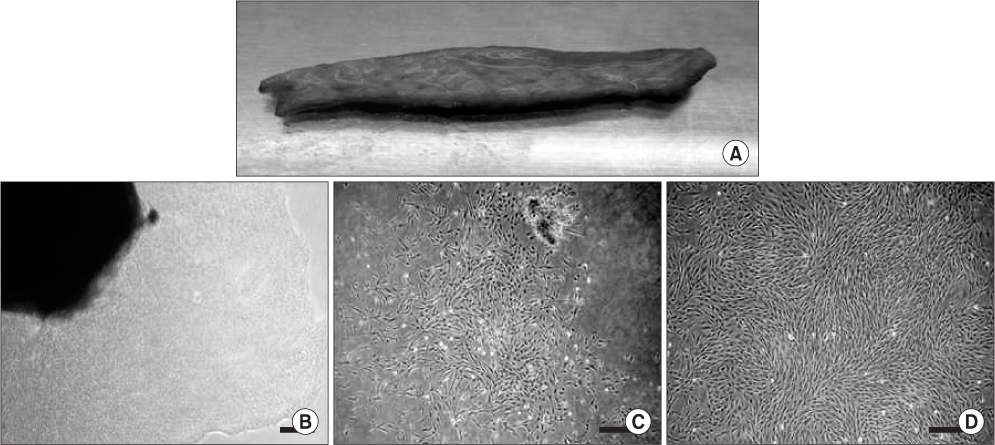
Submandibular skin tissues were harvested from four adult patients and cultured in stem cell media. Isolated hSMSCs were evaluated for their multipotency and other stem cell characteristics. These cells were differentiated into neuronal cells with a chemical induction protocol. During the neuronal induction of hSMSCs, morphological changes and the expression of neuron-specific proteins (by fluorescence-activated cell sorting [FACS]) were evaluated.
RESULTS
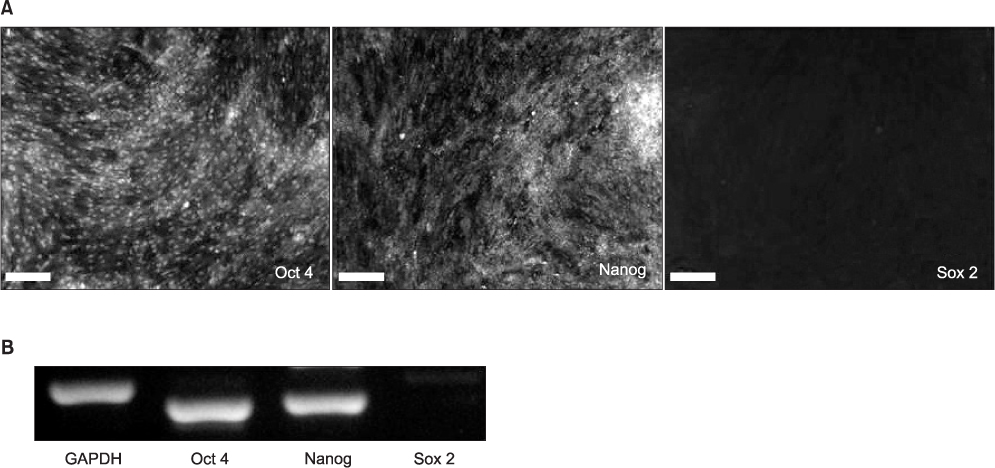
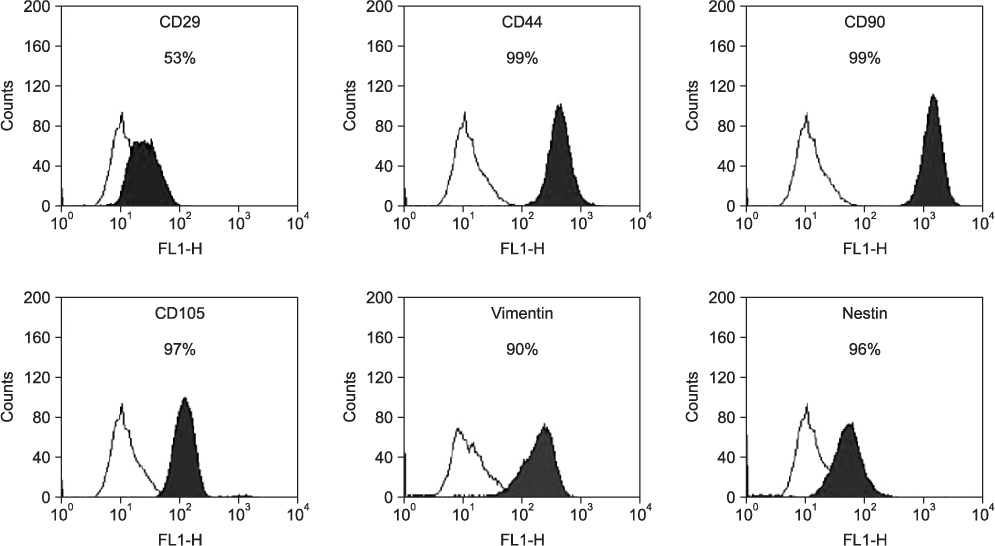
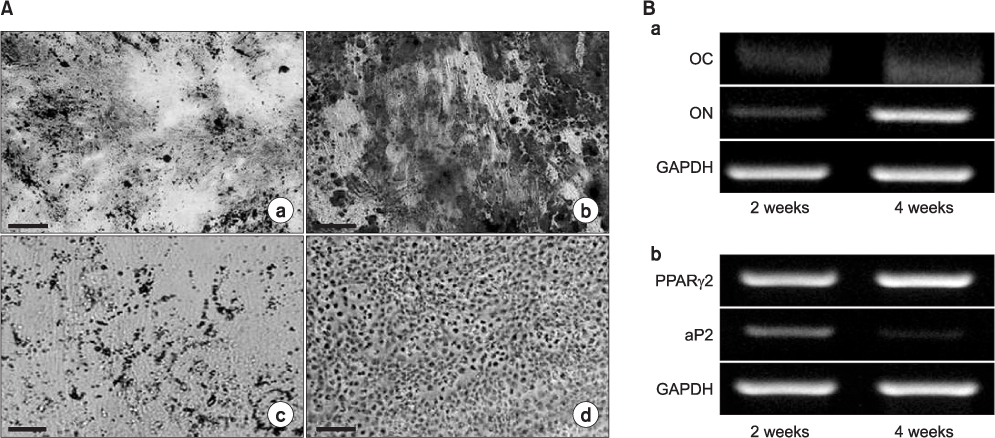
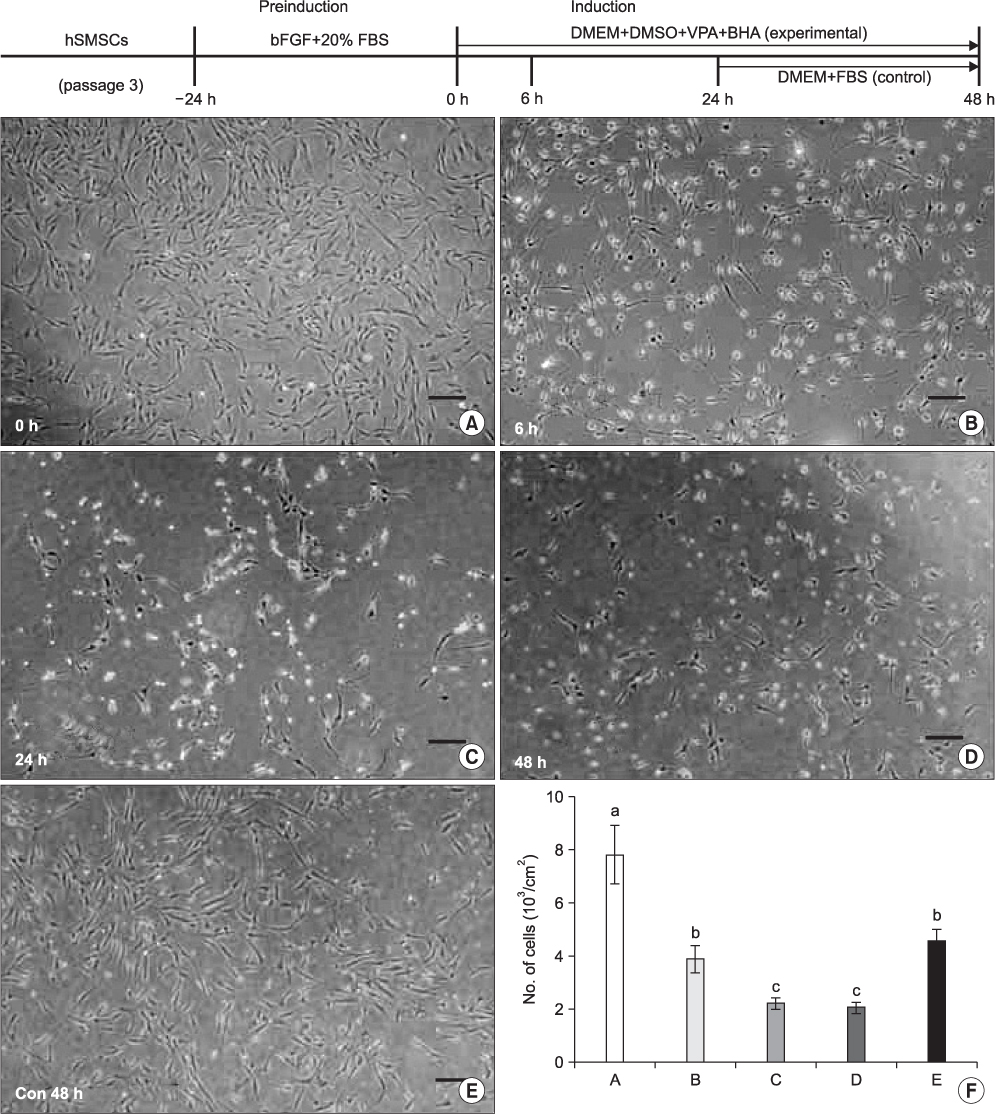
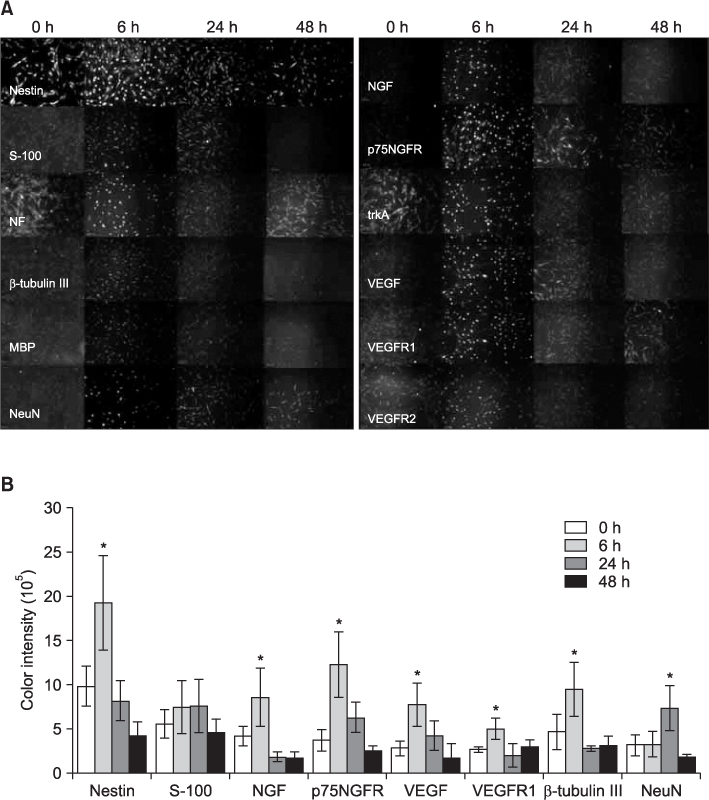
The hSMSCs showed plate-adherence, fibroblast-like growth, expression of the stem-cell transcription factors Oct 4 and Nanog, and positive staining for mesenchymal stem cell (MSC) marker proteins (CD29, CD44, CD90, CD105, and vimentin) and a neural precursor marker (nestin). Moreover, the hSMSCs in this study were successfully differentiated into multiple mesenchymal lineages, including osteocytes, adipocytes, and chondrocytes. Neuron-like cell morphology and various neural markers were highly visible six hours after the neuronal induction of hSMSCs, but their neuron-like characteristics disappeared over time (24-48 hrs). Interestingly, when the chemical induction medium was changed to Dulbecco's Modified Eagle Medium (DMEM) supplemented with fetal bovine serum (FBS), the differentiated cells returned to their hSMSC morphology, and their cell number increased. These results indicate that chemically induced neuron-like cells should not be considered true nerve cells.
CONCLUSION
Isolated hSMSCs have MSC characteristics and express a neural precursor marker, suggesting that human skin is a source of stem cells. However, the in vitro chemical neuronal induction of hSMSC does not produce long-lasting nerve cells and more studies are required before their use in nerve-tissue transplants.
MeSH Terms
Figure
Reference
-
1. Mosahebi A, Woodward B, Wiberg M, Martin R, Terenghi G. Retroviral labeling of Schwann cells: in vitro characterization and in vivo transplantation to improve peripheral nerve regeneration. Glia. 2001. 34:8–17.
Article2. Murakami T, Fujimoto Y, Yasunaga Y, Ishida O, Tanaka N, Ikuta Y, et al. Transplanted neuronal progenitor cells in a peripheral nerve gap promote nerve repair. Brain Res. 2003. 974:17–24.
Article3. Heine W, Conant K, Griffin JW, Höke A. Transplanted neural stem cells promote axonal regeneration through chronically denervated peripheral nerves. Exp Neurol. 2004. 189:231–240.
Article4. Tohill M, Mantovani C, Wiberg M, Terenghi G. Rat bone marrow mesenchymal stem cells express glial markers and stimulate nerve regeneration. Neurosci Lett. 2004. 362:200–203.
Article5. Dezawa M. Systematic neuronal and muscle induction systems in bone marrow stromal cells: the potential for tissue reconstruction in neurodegenerative and muscle degenerative diseases. Med Mol Morphol. 2008. 41:14–19.
Article6. Cuevas P, Carceller F, Garcia-gómez I, Yan M, Dujovny M. Bone marrow stromal cell implantation for peripheral nerve repair. Neurol Res. 2004. 26:230–232.
Article7. Hou SY, Zhang HY, Quan DP, Liu XL, Zhu JK. Tissue-engineered peripheral nerve grafting by differentiated bone marrow stromal cells. Neuroscience. 2006. 140:101–110.
Article8. Toma JG, Akhavan M, Fernandes KJ, Barnabé-heider F, Sadikot A, Kaplan DR, et al. Isolation of multipotent adult stem cells from the dermis of mammalian skin. Nat Cell Biol. 2001. 3:778–784.
Article9. Toma JG, Mckenzie IA, Bagli D, Miller FD. Isolation and characterization of multipotent skin-derived precursors from human skin. Stem Cells. 2005. 23:727–737.
Article10. Shi C, Zhu Y, Su Y, Cheng T. Stem cells and their applications in skin-cell therapy. Trends Biotechnol. 2006. 24:48–52.
Article11. Dyce PW, Zhu H, Craig J, Li J. Stem cells with multilineage potential derived from porcine skin. Biochem Biophys Res Commun. 2004. 316:651–658.
Article12. Blanpain C, Fuchs E. Epidermal stem cells of the skin. Annu Rev Cell Dev Biol. 2006. 22:339–373.
Article13. Ohyama M, Terunuma A, Tock CL, Radonovich MF, Pisemasison CA, Hopping SB, et al. Characterization and isolation of stem cell-enriched human hair follicle bulge cells. J Clin Invest. 2006. 116:249–260.
Article14. Riekstina U, Muceniece R, Cakstina I, Muiznieks I, Ancans J. Characterization of human skin-derived mesenchymal stem cell proliferation rate in different growth conditions. Cytotechnology. 2008. 58:153–162.
Article15. Kang EJ, Byun JH, Choi YJ, Maeng GH, Lee SL, Kang DH, et al. In vitro and in vivo osteogenesis of porcine skin-derived mesenchymal stem cell-like cells with a demineralized bone and fibrin glue scaffold. Tissue Eng Part A. 2010. 16:815–827.
Article16. Woodbury D, Schwarz EJ, Prockop DJ, Black IB. Adult rat and human bone marrow stromal cells differentiate into neurons. J Neurosci Res. 2000. 61:364–370.
Article17. Woodbury D, Reynolds K, Black IB. Adult bone marrow stromal stem cells express germline, ectodermal, endodermal, and mesodermal genes prior to neurogenesis. J Neurosci Res. 2002. 69:908–917.
Article18. Fernandes KJ, Mckenzie IA, Mill P, Smith KM, Akhavan M, Barnabé-heider F, et al. A dermal niche for multipotent adult skin-derived precursor cells. Nat Cell Biol. 2004. 6:1082–1093.
Article19. Fernandes KJ, Kobayashi NR, Gallagher CJ, Barnabé-heider F, Aumont A, Kaplan DR, et al. Analysis of the neurogenic potential of multipotent skin-derived precursors. Exp Neurol. 2006. 201:32–48.
Article20. Hunt DP, Morris PN, Sterling J, Anderson JA, Joannides A, Jahoda C, et al. A highly enriched niche of precursor cells with neuronal and glial potential within the hair follicle dermal papilla of adult skin. Stem Cells. 2008. 26:163–172.
Article21. Zhao M, Isom SC, Lin H, Hao Y, Zhang Y, Zhao J, et al. Tracing the stemness of porcine skin-derived progenitors (pSKP) back to specific marker gene expression. Cloning Stem Cells. 2009. 11:111–122.
Article22. Shih DT, Lee DC, Chen SC, Tsai RY, Huang CT, Tsai CC, et al. Isolation and characterization of neurogenic mesenchymal stem cells in human scalp tissue. Stem Cells. 2005. 23:1012–1020.
Article23. Marchesi C, Pluderi M, Colleoni F, Belicchi M, Meregalli M, Farini A, et al. Skin-derived stem cells transplanted into resorbable guides provide functional nerve regeneration after sciatic nerve resection. Glia. 2007. 55:425–438.
Article24. Riekstina U, Cakstina I, Parfejevs V, Hoogduijn M, Jankovskis G, Muiznieks I, et al. Embryonic stem cell marker expression pattern in human mesenchymal stem cells derived from bone marrow, adipose tissue, heart and dermis. Stem Cell Rev. 2009. 5:378–386.
Article25. Sanchez-ramos J, Song S, Cardozo-pelaez F, Hazzi C, Stedeford T, Willing A, et al. Adult bone marrow stromal cells differentiate into neural cells in vitro. Exp Neurol. 2000. 164:247–256.
Article26. Kim BJ, Seo JH, Bubien JK, Oh YS. Differentiation of adult bone marrow stem cells into neuroprogenitor cells in vitro. Neuroreport. 2002. 13:1185–1188.
Article27. Deng W, Obrocka M, Fischer I, Prockop DJ. In vitro differentiation of human marrow stromal cells into early progenitors of neural cells by conditions that increase intracellular cyclic AMP. Biochem Biophys Res Commun. 2001. 282:148–152.
Article28. Wang FW, Jia DY, Du ZH, Fu J, Zhao SD, Liu SM, et al. Roles of activated astrocytes in bone marrow stromal cell proliferation and differentiation. Neuroscience. 2009. 160:319–329.
Article29. Bossolasco P, Cova L, Calzarossa C, Rimoldi SG, Borsotti C, Deliliers GL, et al. Neuro-glial differentiation of human bone marrow stem cells in vitro. Exp Neurol. 2005. 193:312–325.
Article30. Lei Z, Yongda L, Jun M, Yingyu S, Shaoju Z, Xinwen Z, et al. Culture and neural differentiation of rat bone marrow mesenchymal stem cells in vitro. Cell Biol Int. 2007. 31:916–923.
Article31. Yamaguchi S, Kuroda S, Kobayashi H, Shichinohe H, Yano S, Hida K, et al. The effects of neuronal induction on gene expression profile in bone marrow stromal cells (BMSC)--a preliminary study using microarray analysis. Brain Res. 2006. 1087:15–27.
Article32. Muñoz-elías G, Woodbury D, Black IB. Marrow stromal cells, mitosis, and neuronal differentiation: stem cell and precursor functions. Stem Cells. 2003. 21:437–448.
Article33. Barnabé GF, Schwindt TT, Calcagnotto ME, Motta FL, Martinez GJ, De OA, et al. Chemically-induced RAT mesenchymal stem cells adopt molecular properties of neuronal-like cells but do not have basic neuronal functional properties. PLoS One. 2009. 4:e5222.
Article34. Lu P, Blesch A, Tuszynski MH. Induction of bone marrow stromal cells to neurons: differentiation, transdifferentiation, or artifact? J Neurosci Res. 2004. 77:174–191.
Article35. Neuhuber B, Gallo G, Howard L, Kostura L, Mackay A, Fischer I. Reevaluation of in vitro differentiation protocols for bone marrow stromal cells: disruption of actin cytoskeleton inducesrapid morphological changes and mimics neuronal phenotype. J Neurosci Res. 2004. 77:192–204.
Article36. Rismanchi N, Floyd CL, Berman RF, Lyeth BG. Cell death and long-term maintenance of neuron-like state after differentiation of rat bone marrow stromal cells: a comparison of protocols. Brain Res. 2003. 991:46–55.
Article37. Rho GJ, Kumar BM, Balasubramanian SS. Porcine mesenchymal stem cells--current technological status and future perspective. Front Biosci. 2009. 14:3942–3961.38. Shimizu S, Kitada M, Ishikawa H, Itokazu Y, Wakao S, Dezawa M. Peripheral nerve regeneration by the in vitro differentiated-human bone marrow stromal cells with Schwann cell property. Biochem Biophys Res Commun. 2007. 359:915–920.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Concise Review: Differentiation of Human Adult Stem Cells Into Hepatocyte-like Cells In vitro
- Adipose Tissue Derived Mesenchymal Stem Cells
- In vitro neuronal and osteogenic differentiation of mesenchymal stem cells from human umbilical cord blood
- Comparative Evaluation for Potential Differentiation of Endothelial Progenitor Cells and Mesenchymal Stem Cells into Endothelial-Like Cells
- Differentiation of adipose-derived stem cells into Schwann-like cells: fetal bovine serum or human serum?