Brain Tumor Res Treat.
2014 Oct;2(2):114-118. 10.14791/btrt.2014.2.2.114.
Isolated Central Nervous System Relapse of Acute Lymphoblastic Leukemia
- Affiliations
-
- 1Department of Neurosurgery, Korea Cancer Center Hospital, Korea Institute of Radiological and Medical Science, Seoul, Korea. 20100049@kirams.re.kr
- KMID: 2134282
- DOI: http://doi.org/10.14791/btrt.2014.2.2.114
Abstract
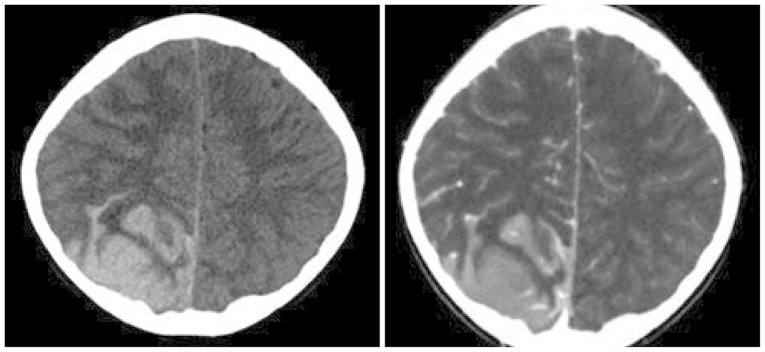
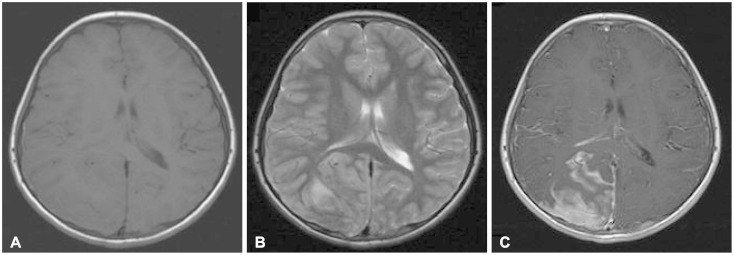
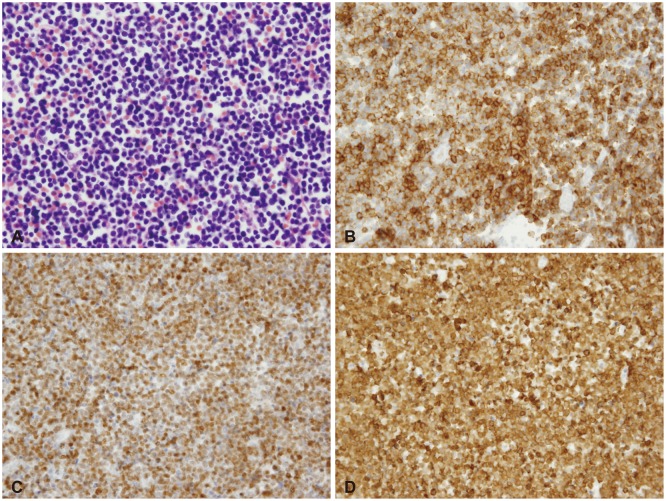
- Acute lymphoblastic leukemia (ALL) is the most common form of childhood cancer and may exhibit central nervous system (CNS) involvement. Advances in chemotherapy and effective CNS prophylaxis have significantly decreased the incidence of CNS relapse of ALL to 5-10%. Here, we report the case of a patient with isolated CNS relapse of standard risk group pre-B-cell type ALL in an 11-year-old girl, relapsed 3 years after successful completion of chemotherapy. An 11-year-old girl visited our hospital complaining of headache, dizziness, vomiting, and visual field defects. Neurological examination revealed left-side homonymous hemianopsia. Brain magnetic resonance imaging showed a large irregular dural-based sulcal hematoma in the right parietal and occipital lobes. Surgery to remove the hematoma revealed the existence of hematopoietic malignancy after pathologic evaluation. Bone marrow biopsy was subsequently performed but showed no evidence of malignancy.
Keyword
MeSH Terms
-
Biopsy
Bone Marrow
Brain
Central Nervous System*
Child
Dizziness
Drug Therapy
Female
Headache
Hematologic Neoplasms
Hematoma
Hemianopsia
Humans
Incidence
Leukemia
Magnetic Resonance Imaging
Neurologic Examination
Occipital Lobe
Precursor Cell Lymphoblastic Leukemia-Lymphoma*
Precursor Cells, B-Lymphoid
Rabeprazole
Recurrence*
Visual Fields
Vomiting
Figure
Reference
-
1. Badr MA, Hassan TH, El-Gerby KM, Lamey ME. Magnetic resonance imaging of the brain in survivors of childhood acute lymphoblastic leukemia. Oncol Lett. 2013; 5:621–626. PMID: 23420690.
Article2. Schroeder H, Garwicz S, Kristinsson J, Siimes MA, Wesenberg F, Gustafsson G. Outcome after first relapse in children with acute lymphoblastic leukemia: a population-based study of 315 patients from the Nordic Society of Pediatric Hematology and Oncology (NOPHO). Med Pediatr Oncol. 1995; 25:372–378. PMID: 7674994.
Article3. Barredo J, Ritchey AK. Controversies in the management of central nervous system leukemia. Pediatr Hematol Oncol. 2010; 27:329–332. PMID: 20469977.
Article4. Pui CH. Central nervous system disease in acute lymphoblastic leukemia: prophylaxis and treatment. Hematology Am Soc Hematol Educ Program. 2006; 142–146. PMID: 17124053.
Article5. Chan MS, Roebuck DJ, Yuen MP, Li CK, Chan YL. MR imaging of the brain in patients cured of acute lymphoblastic leukemia--the value of gradient echo imaging. AJNR Am J Neuroradiol. 2006; 27:548–552. PMID: 16551991.6. Gay CT, Bodensteiner JB, Nitschke R, Sexauer C, Wilson D. Reversible treatment-related leukoencephalopathy. J Child Neurol. 1989; 4:208–213. PMID: 2768785.
Article7. Pui CH, Campana D, Pei D, et al. Treating childhood acute lymphoblastic leukemia without cranial irradiation. N Engl J Med. 2009; 360:2730–2741. PMID: 19553647.
Article8. Mitchell CD, Richards SM, Kinsey SE, et al. Benefit of dexamethasone compared with prednisolone for childhood acute lymphoblastic leukaemia: results of the UK Medical Research Council ALL97 randomized trial. Br J Haematol. 2005; 129:734–745. PMID: 15952999.
Article9. Seibel NL, Steinherz PG, Sather HN, et al. Early postinduction intensification therapy improves survival for children and adolescents with high-risk acute lymphoblastic leukemia: a report from the Children's Oncology Group. Blood. 2008; 111:2548–2555. PMID: 18039957.10. Nguyen K, Devidas M, Cheng SC, et al. Factors influencing survival after relapse from acute lymphoblastic leukemia: a Childrens Oncology Group study. Leukemia. 2008; 22:2142–2150. PMID: 18818707.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- An Isolated Relapse of Acute Lymphoblastic Leukemia as a Chest Wall Mass
- An Unusual Relapse of Acute Lymphoblastic Leukemia in the Uterine Corpus
- Isolated Breast Relapse of Early T-Cell Precursor Acute Lymphoblastic Leukemia after Stem Cell Transplantation: A Pediatric Case and Literature Review
- Intermittent central nervous system irradiation and intrathecal chemotherapy for recurrent central nervous system leukemia in children
- The CNS Relapse of the Acute Lymphoblastic Leukemia: Radiotherapy Results