Anat Cell Biol.
2012 Dec;45(4):241-258. 10.5115/acb.2012.45.4.241.
Nuclear expression of PG-21, SRC-1, and pCREB in regions of the lumbosacral spinal cord involved in pelvic innervation in young adult and aged rats
- Affiliations
-
- 1Cardiff School of Biosciences, Cardiff University, Cardiff, UK. santer@cardiff.ac.uk
- 2School of Applied Sciences, Northumbria University, Newcastle upon Tyne, UK.
- KMID: 2133910
- DOI: http://doi.org/10.5115/acb.2012.45.4.241
Abstract
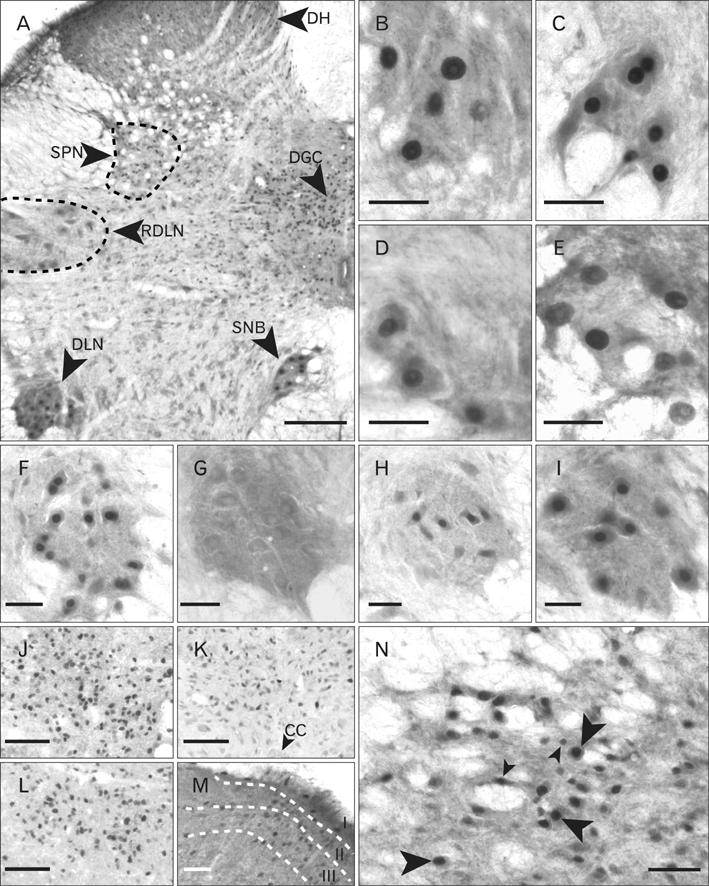
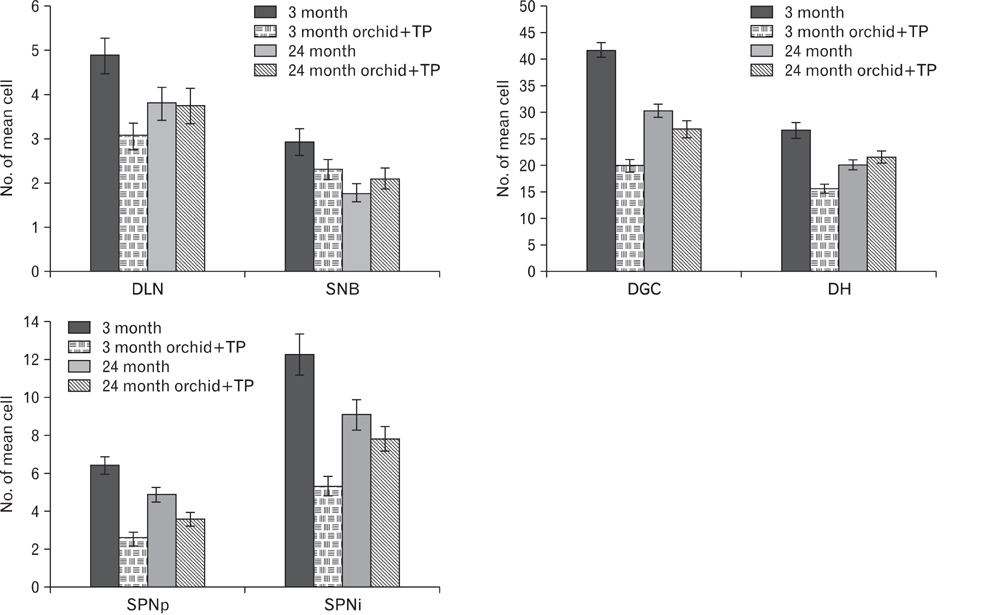
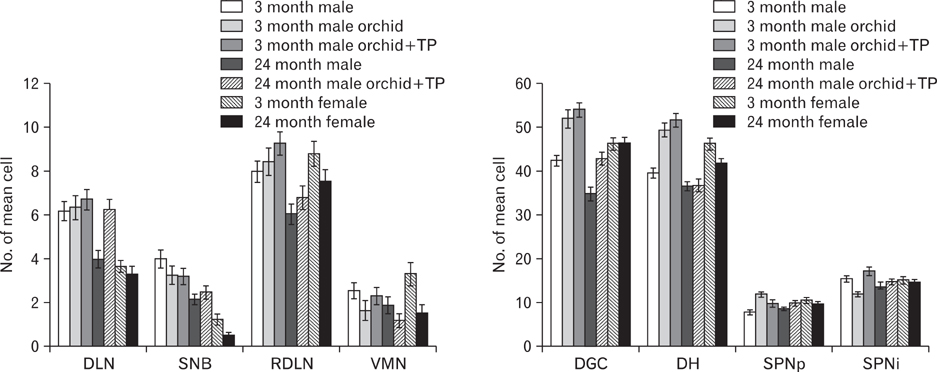
- In rats, ageing results in dysfunctional patterns of micturition and diminished sexual reflexes that may reflect degenerative changes within spinal circuitry. In both sexes the dorsal lateral nucleus and the spinal nucleus of the bulbospongiosus, which lie in the L5-S1 spinal segments, contain motor neurons that innervate perineal muscles, and the external anal and urethral sphincters. Neurons in the sacral parasympathetic nucleus of these segments provide autonomic control of the bladder, cervix and penis and other lower urinary tract structures. Interneurons in the dorsal gray commissure and dorsal horn have also been implicated in lower urinary tract function. This study investigates the cellular localisation of PG-21 androgen receptors, steroid receptor co-activator one (SRC-1) and the phosphorylated form of c-AMP response element binding protein (pCREB) within these spinal nuclei. These are components of signalling pathways that mediate cellular responses to steroid hormones and neurotrophins. Nuclear expression of PG-21 androgen receptors, SRC-1 and pCREB in young and aged rats was quantified using immunohistochemistry. There was a reduction in the number of spinal neurons expressing these molecules in the aged males while in aged females, SRC-1 and pCREB expression was largely unchanged. This suggests that the observed age-related changes may be linked to declining testosterone levels. Acute testosterone therapy restored expression of PG-21 androgen receptor in aged and orchidectomised male rats, however levels of re-expression varied within different nuclei suggesting a more prolonged period of hormone replacement may be required for full restoration.
MeSH Terms
-
Aged
Androgens
Animals
Carrier Proteins
Cervix Uteri
Female
Hormone Replacement Therapy
Horns
Humans
Immunohistochemistry
Interneurons
Male
Motor Neurons
Muscles
Nerve Growth Factors
Neurons
Penis
Rats
Receptors, Androgen
Receptors, Steroid
Reflex
Response Elements
Spinal Cord
Testosterone
Urethra
Urinary Bladder
Urinary Tract
Urination
Young Adult
Androgens
Carrier Proteins
Nerve Growth Factors
Receptors, Androgen
Receptors, Steroid
Testosterone
Figure
Reference
-
1. Dering MA, Santer RM, Watson AH. Age-related changes in the morphology of preganglionic neurons projecting to the rat hypogastric ganglion. J Neurocytol. 1996. 25:555–563.2. Hancock MB, Peveto CA. Preganglionic neurons in the sacral spinal cord of the rat: an HRP study. Neurosci Lett. 1979. 11:1–5.3. Nadelhaft I, Booth AM. The location and morphology of preganglionic neurons and the distribution of visceral afferents from the rat pelvic nerve: a horseradish peroxidase study. J Comp Neurol. 1984. 226:238–245.4. Ranson RN, Santer RM, Watson AH. The relationship between serotonin, dopamine beta hydroxylase and GABA immunoreactive inputs and spinal preganglionic neurones projecting to the major pelvic ganglion of Wistar rats. Neuroscience. 2006. 141:1935–1949.5. Santer RM, Dering MA, Ranson RN, Waboso HN, Watson AH. Differential susceptibility to ageing of rat preganglionic neurones projecting to the major pelvic ganglion and of their afferent inputs. Auton Neurosci. 2002. 96:73–81.6. Yaici ED, Rampin O, Tang Y, Calas A, Jestin A, Leclerc P, Benoit G, Giuliano F. Catecholaminergic projections onto spinal neurons destined to the pelvis including the penis in rat. Int J Impot Res. 2002. 14:151–166.7. Dering MA, Santer RM, Watson AH. Age-related changes in the morphology of preganglionic neurons projecting to the paracervical ganglion of nulliparous and multiparous rats. Brain Res. 1998. 780:245–252.8. Papka RE, Newton BW, McNeill DL. Origin of galanin-immunoreactive nerve fibers in the rat paracervical autonomic ganglia and uterine cervix. J Auton Nerv Syst. 1991. 33:25–33.9. Dail WG, Trujillo D, de la Rosa D, Walton G. Autonomic innervation of reproductive organs: analysis of the neurons whose axons project in the main penile nerve in the pelvic plexus of the rat. Anat Rec. 1989. 224:94–101.10. Kepper M, Keast J. Immunohistochemical properties and spinal connections of pelvic autonomic neurons that innervate the rat prostate gland. Cell Tissue Res. 1995. 281:533–542.11. Papka RE, Cotton JP, Traurig HH. Comparative distribution of neuropeptide tyrosine-, vasoactive intestinal polypeptide-, substance P-immunoreactive, acetylcholinesterase-positive and noradrenergic nerves in the reproductive tract of the female rat. Cell Tissue Res. 1985. 242:475–490.12. Papka RE, McCurdy JR, Williams SJ, Mayer B, Marson L, Platt KB. Parasympathetic preganglionic neurons in the spinal cord involved in uterine innervation are cholinergic and nitric oxide-containing. Anat Rec. 1995. 241:554–562.13. Papka RE, Traurig HH, Schemann M, Collins J, Copelin T, Wilson K. Cholinergic neurons of the pelvic autonomic ganglia and uterus of the female rat: distribution of axons and presence of muscarinic receptors. Cell Tissue Res. 1999. 296:293–305.14. Purinton PT, Fletcher TF, Bradley WE. Gross and light microscopic features of the pelvic plexus in the rat. Anat Rec. 1973. 175:697–705.15. Marson L. Identification of central nervous system neurons that innervate the bladder body, bladder base, or external urethral sphincter of female rats: a transneuronal tracing study using pseudorabies virus. J Comp Neurol. 1997. 389:584–602.16. Nadelhaft I, Miranda-Sousa AJ, Vera PL. Separate urinary bladder and prostate neurons in the central nervous system of the rat: simultaneous labeling with two immunohistochemically distinguishable pseudorabies viruses. BMC Neurosci. 2002. 3:8.17. Vera PL, Nadelhaft I. Anatomical evidence for two spinal 'afferent-interneuron-efferent' reflex pathways involved in micturition in the rat: a 'pelvic nerve' reflex pathway and a 'sacrolumbar intersegmental' reflex pathway. Brain Res. 2000. 883:107–118.18. Marson L, Cai R, Makhanova N. Identification of spinal neurons involved in the urethrogenital reflex in the female rat. J Comp Neurol. 2003. 462:355–370.19. Marson L, Murphy AZ. Identification of neural circuits involved in female genital responses in the rat: a dual virus and anterograde tracing study. Am J Physiol Regul Integr Comp Physiol. 2006. 291:R419–R428.20. Marson L, Carson CC 3rd. Central nervous system innervation of the penis, prostate, and perineal muscles: a transneuronal tracing study. Mol Urol. 1999. 3:43–50.21. Marson L, Gravitt K. Spinal neurons activated with the urethrogenital reflex in the male rat. Brain Res. 2004. 1026:108–115.22. Yaïci ED, Rampin O, Calas A, Jestin A, McKenna KE, Leclerc P, Benoit G, Giuliano F. alpha(2a) and alpha(2c) adrenoceptors on spinal neurons controlling penile erection. Neuroscience. 2002. 114:945–960.23. Berkley KJ, Hubscher CH, Wall PD. Neuronal responses to stimulation of the cervix, uterus, colon, and skin in the rat spinal cord. J Neurophysiol. 1993. 69:545–556.24. Blok BF. Central pathways controlling micturition and urinary continence. Urology. 2002. 59:5 Suppl 1. 13–17.25. Giuliano F, Rampin O. Neural control of erection. Physiol Behav. 2004. 83:189–201.26. Jordan CL, Breedlove SM, Arnold AP. Sexual dimorphism and the influence of neonatal androgen in the dorsolateral motor nucleus of the rat lumbar spinal cord. Brain Res. 1982. 249:309–314.27. McKenna KE, Nadelhaft I. The organization of the pudendal nerve in the male and female rat. J Comp Neurol. 1986. 248:532–549.28. Schrøder HD. Organization of the motoneurons innervating the pelvic muscles of the male rat. J Comp Neurol. 1980. 192:567–587.29. Holmes GM, Rogers RC, Bresnahan JC, Beattie MS. External anal sphincter hyperreflexia following spinal transection in the rat. J Neurotrauma. 1998. 15:451–457.30. Fraser MO, Chancellor MB. Neural control of the urethra and development of pharmacotherapy for stress urinary incontinence. BJU Int. 2003. 91:743–748.31. Nordeen EJ, Nordeen KW, Sengelaub DR, Arnold AP. Androgens prevent normally occurring cell death in a sexually dimorphic spinal nucleus. Science. 1985. 229:671–673.32. Sengelaub DR, Arnold AP. Hormonal control of neuron number in sexually dimorphic spinal nuclei of the rat: I. Testosterone-regulated death in the dorsolateral nucleus. J Comp Neurol. 1989. 280:622–629.33. Breedlove SM, Arnold AP. Hormone accumulation in a sexually dimorphic motor nucleus of the rat spinal cord. Science. 1980. 210:564–566.34. Breedlove SM, Arnold AP. Sexually dimorphic motor nucleus in the rat lumbar spinal cord: response to adult hormone manipulation, absence in androgen-insensitive rats. Brain Res. 1981. 225:297–307.35. Tobin AM, Payne AP. Perinatal androgen administration and the maintenance of sexually dimorphic and nondimorphic lumbosacral motor neuron groups in female Albino Swiss rats. J Anat. 1991. 177:47–53.36. Leslie M, Forger NG, Breedlove SM. Sexual dimorphism and androgen effects on spinal motoneurons innervating the rat flexor digitorum brevis. Brain Res. 1991. 561:269–273.37. Nicolopoulos-Stournaras S, Iles JF. Motor neuron columns in the lumbar spinal cord of the rat. J Comp Neurol. 1983. 217:75–85.38. Hodges LL, Jordan CL, Breedlove SM. Hormone-sensitive periods for the control of motoneuron number and soma size in the dorsolateral nucleus of the rat spinal cord. Brain Res. 1993. 602:187–190.39. Kurz EM, Brewer RG, Sengelaub DR. Hormonally mediated plasticity of motoneuron morphology in the adult rat spinal cord: a cholera toxin-HRP study. J Neurobiol. 1991. 22:976–988.40. Goldstein LA, Kurz EM, Sengelaub DR. Androgen regulation of dendritic growth and retraction in the development of a sexually dimorphic spinal nucleus. J Neurosci. 1990. 10:935–946.41. Goldstein LA, Sengelaub DR. Motoneuron morphology in the dorsolateral nucleus of the rat spinal cord: normal development and androgenic regulation. J Comp Neurol. 1993. 338:588–600.42. Fargo KN, Iwema CL, Clark-Phelps MC, Sengelaub DR. Exogenous testosterone reverses age-related atrophy in a spinal neuromuscular system. Horm Behav. 2007. 51:20–30.43. Matsumoto A. Synaptic changes in the perineal motoneurons of aged male rats. J Comp Neurol. 1998. 400:103–109.44. Ranson RN, Santer RM, Watson AH. Ageing reduces the number of vesicular glutamate transporter 2 containing immunoreactive inputs to identified rat pelvic motoneurons. Exp Gerontol. 2007. 42:506–516.45. Ghanadian R, Lewis JG, Chisholm GD. Serum testosterone and dihydrotestosterone changes with age in rat. Steroids. 1975. 25:753–762.46. Matsumoto A, Prins GS. Age-dependent changes in androgen receptor immunoreactivity in motoneurons of the spinal nucleus of the bulbocavernosus of male rats. Neurosci Lett. 1998. 243:29–32.47. Matsumoto A, Prins GS. Androgenic regulation of expression of androgen receptor protein in the perineal motoneurons of aged male rats. J Comp Neurol. 2002. 443:383–387.48. Smith ER, Stefanick ML, Clark JT, Davidson JM. Hormones and sexual behavior in relationship to aging in male rats. Horm Behav. 1992. 26:110–135.49. McKenna NJ, O'Malley BW. Combinatorial control of gene expression by nuclear receptors and coregulators. Cell. 2002. 108:465–474.50. Shibata H, Spencer TE, Oñate SA, Jenster G, Tsai SY, Tsai MJ, O'Malley BW. Role of co-activators and co-repressors in the mechanism of steroid/thyroid receptor action. Recent Prog Horm Res. 1997. 52:141–164.51. Leo C, Chen JD. The SRC family of nuclear receptor coactivators. Gene. 2000. 245:1–11.52. Smith CL, Oñate SA, Tsai MJ, O'Malley BW. CREB binding protein acts synergistically with steroid receptor coactivator-1 to enhance steroid receptor-dependent transcription. Proc Natl Acad Sci U S A. 1996. 93:8884–8888.53. Wu RC, Smith CL, O'Malley BW. Transcriptional regulation by steroid receptor coactivator phosphorylation. Endocr Rev. 2005. 26:393–399.54. McManus KJ, Hendzel MJ. CBP, a transcriptional coactivator and acetyltransferase. Biochem Cell Biol. 2001. 79:253–266.55. Gonzalez GA, Montminy MR. Cyclic AMP stimulates somatostatin gene transcription by phosphorylation of CREB at serine 133. Cell. 1989. 59:675–680.56. Yamamoto KK, Gonzalez GA, Biggs WH 3rd, Montminy MR. Phosphorylation-induced binding and transcriptional efficacy of nuclear factor CREB. Nature. 1988. 334:494–498.57. Montminy MR, Gonzalez GA, Yamamoto KK. Regulation of cAMP-inducible genes by CREB. Trends Neurosci. 1990. 13:184–188.58. Finkbeiner S. CREB couples neurotrophin signals to survival messages. Neuron. 2000. 25:11–14.59. Reichardt LF. Neurotrophin-regulated signalling pathways. Philos Trans R Soc Lond B Biol Sci. 2006. 361:1545–1564.60. Mattson MP, Chan SL, Duan W. Modification of brain aging and neurodegenerative disorders by genes, diet, and behavior. Physiol Rev. 2002. 82:637–672.61. Ulfhake B, Bergman E, Edstrom E, Fundin BT, Johnson H, Kullberg S, Ming Y. Regulation of neurotrophin signaling in aging sensory and motoneurons: dissipation of target support? Mol Neurobiol. 2000. 21:109–135.62. Watson FL, Heerssen HM, Bhattacharyya A, Klesse L, Lin MZ, Segal RA. Neurotrophins use the Erk5 pathway to mediate a retrograde survival response. Nat Neurosci. 2001. 4:981–988.63. Jordan C. Androgen receptor (AR) immunoreactivity in rat pudendal motoneurons: implications for accessory proteins. Horm Behav. 1997. 32:1–10.64. Jordan CL, Padgett B, Hershey J, Prins G, Arnold A. Ontogeny of androgen receptor immunoreactivity in lumbar motoneurons and in the sexually dimorphic levator ani muscle of male rats. J Comp Neurol. 1997. 379:88–98.65. Lubischer JL, Arnold AP. Axotomy transiently down-regulates androgen receptors in motoneurons of the spinal nucleus of the bulbocavernosus. Brain Res. 1995. 694:61–68.66. Matsumoto A. Age-related changes in nuclear receptor coactivator immunoreactivity in motoneurons of the spinal nucleus of the bulbocavernosus of male rats. Brain Res. 2002. 943:202–205.67. Matsumoto A, Arai Y, Prins GS. Androgenic regulation of androgen receptor immunoreactivity in motoneurons of the spinal nucleus of the bulbocavernosus of male rats. J Neuroendocrinol. 1996. 8:553–559.68. Watkins TW, Keast JR. Androgen-sensitive preganglionic neurons innervate the male rat pelvic ganglion. Neuroscience. 1999. 93:1147–1157.69. O'Bryant EL, Jordan CL. Expression of nuclear receptor coactivators in androgen-responsive and -unresponsive motoneurons. Horm Behav. 2005. 47:29–38.70. Ranson RN, Priestley DJ, Santer RM, Watson AH. Changes in the substance P-containing innervation of the lumbosacral spinal cord in male Wistar rats as a consequence of ageing. Brain Res. 2005. 1036:139–144.71. Ranson RN, Santer RM, Watson AH. SRC-1 localisation in lumbosacral spinal cord of male and female Wistar rats. Neuroreport. 2003. 14:1821–1824.72. Matsumoto A. Age-dependent changes in phosphorylated cAMP response element-binding protein immunoreactivity in motoneurons of the spinal nucleus of the bulbocavernosus of male rats. Neurosci Lett. 2000. 279:117–120.73. Gréco B, Edwards DA, Zumpe D, Michael RP, Clancy AN. Fos induced by mating or noncontact sociosexual interaction is colocalized with androgen receptors in neurons within the forebrain, midbrain, and lumbosacral spinal cord of male rats. Horm Behav. 1998. 33:125–138.74. Nadelhaft I, Vera PL. Central nervous system neurons infected by pseudorabies virus injected into the rat urinary bladder following unilateral transection of the pelvic nerve. J Comp Neurol. 1995. 359:443–456.75. Tang Y, Rampin O, Giuliano F, Ugolini G. Spinal and brain circuits to motoneurons of the bulbospongiosus muscle: retrograde transneuronal tracing with rabies virus. J Comp Neurol. 1999. 414:167–192.76. Eika B, Levin RM, Longhurst PA. Modulation of urinary bladder function by sex hormones in streptozotocin-diabetic rats. J Urol. 1994. 152(2 Pt 1):537–543.77. Collins WF 3rd, Seymour AW, Klugewicz SW. Differential effect of castration on the somal size of pudendal motoneurons in the adult male rat. Brain Res. 1992. 577:326–330.78. Chambers KC, Phoenix CH. Testosterone and the decline of sexual behavior in aging male rats. Behav Neural Biol. 1984. 40:87–97.79. Chambers KC, Phoenix CH. Testosterone is more effective than dihydrotestosterone plus estradiol in activating sexual behavior in old male rats. Neurobiol Aging. 1986. 7:127–132.80. Davidson JM, Stefanick ML, Sachs BD, Smith ER. Role of androgen in sexual reflexes of the male rat. Physiol Behav. 1978. 21:141–146.81. Frankel AI, Mock EJ. Time course of hormonal response to sexual behavior in aging male rats. Exp Gerontol. 1981. 16:363–369.82. Gray GD. Age-related changes in penile erections and circulating testosterone in middle-aged male rats. Adv Exp Med Biol. 1978. 113:149–158.83. Meisel RL, O'Hanlon JK, Sachs BD. Differential maintenance of penile responses and copulatory behavior by gonadal hormones in castrated male rats. Horm Behav. 1984. 18:56–64.84. Hsu HK, Hsu C, Yu JY, Peng MT. Effects of long-term testosterone replacement on copulatory activity in old male rats. Gerontology. 1986. 32:10–17.85. Ranson RN, Dodds AL, Smith MJ, Santer RM, Watson AH. Age-associated changes in the monoaminergic innervation of rat lumbosacral spinal cord. Brain Res. 2003. 972:149–158.86. Amandusson A, Hermanson O, Blomqvist A. Estrogen receptor-like immunoreactivity in the medullary and spinal dorsal horn of the female rat. Neurosci Lett. 1995. 196:25–28.87. Papka RE, Storey-Workley M, Shughrue PJ, Merchenthaler I, Collins JJ, Usip S, Saunders PT, Shupnik M. Estrogen receptor-alpha and beta- immunoreactivity and mRNA in neurons of sensory and autonomic ganglia and spinal cord. Cell Tissue Res. 2001. 304:193–214.88. VanderHorst VG, Meijer E, Holstege G. Estrogen receptor-alpha immunoreactivity in parasympathetic preganglionic neurons innervating the bladder in the adult ovariectomized cat. Neurosci Lett. 2001. 298:147–150.89. Williams SJ, Papka RE. Estrogen receptor-immunoreactive neurons are present in the female rat lumbosacral spinal cord. J Neurosci Res. 1996. 46:492–501.90. Burke KA, Schroeder DM, Abel RA, Richardson SC, Bigsby RM, Nephew KP. Immunohistochemical detection of estrogen receptor alpha in male rat spinal cord during development. J Neurosci Res. 2000. 61:329–337.91. Blanco CE, Peltz A, Staley R, Kim F. Effects of pharmacologic androgen treatment duration on glucocorticoid receptor alpha immunoreactivity of lumbosacral motor neurons in the male rat. Neuroscience. 2002. 115:941–949.92. Ferrini M, Gonzalez S, Antakly T, De Nicola AF. Immunocytochemical localization of glucocorticoid receptors in the spinal cord: effects of adrenalectomy, glucocorticoid treatment, and spinal cord transection. Cell Mol Neurobiol. 1993. 13:387–397.93. Yan P, Xu J, Li Q, Chen S, Kim GM, Hsu CY, Xu XM. Glucocorticoid receptor expression in the spinal cord after traumatic injury in adult rats. J Neurosci. 1999. 19:9355–9363.94. Labombarda F, Guennoun R, Gonzalez S, Roig P, Lima A, Schumacher M, De Nicola AF. Immunocytochemical evidence for a progesterone receptor in neurons and glial cells of the rat spinal cord. Neurosci Lett. 2000. 288:29–32.95. Monks DA, Arciszewska G, Watson NV. Estrogen-inducible progesterone receptors in the rat lumbar spinal cord: regulation by ovarian steroids and fluctuation across the estrous cycle. Horm Behav. 2001. 40:490–496.96. Blanchet P, Yaici el D, Cayzergues L, Giuliano F, Jardin A, Benoit G, Droupy S. Identification of androgen receptors in the motoneurons of the external urethral sphincter in the spinal cord of female rats. Eur Urol. 2005. 47:118–124.97. Ranson RN, Santer RM, Watson AH. Biogenic amine and neuropeptide inputs to identified pelvic floor motoneurons that also express SRC-1. Neurosci Lett. 2005. 382:248–253.98. Monks DA, Xu J, O'Malley BW, Jordan CL. Steroid receptor coactivator-1 is not required for androgen-mediated sexual differentiation of spinal motoneurons. Neuroendocrinology. 2003. 78:45–51.99. Balazs R. Trophic effect of glutamate. Curr Top Med Chem. 2006. 6:961–968.100. De Cesare D, Fimia GM, Sassone-Corsi P. Signaling routes to CREM and CREB: plasticity in transcriptional activation. Trends Biochem Sci. 1999. 24:281–285.101. Mayr B, Montminy M. Transcriptional regulation by the phosphorylation-dependent factor CREB. Nat Rev Mol Cell Biol. 2001. 2:599–609.102. Tan Y, Rouse J, Zhang A, Cariati S, Cohen P, Comb MJ. FGF and stress regulate CREB and ATF-1 via a pathway involving p38 MAP kinase and MAPKAP kinase-2. EMBO J. 1996. 15:4629–4642.103. Ernfors P, Bramham CR. The coupling of a trkB tyrosine residue to LTP. Trends Neurosci. 2003. 26:171–173.104. Osborne MC, Verhovshek T, Sengelaub DR. Androgen regulates trkB immunolabeling in spinal motoneurons. J Neurosci Res. 2007. 85:303–309.105. Johnson H, Hokfelt T, Ulfhake B. Decreased expression of TrkB and TrkC mRNAs in spinal motoneurons of aged rats. Eur J Neurosci. 1996. 8:494–499.106. Yang LY, Arnold AP. Interaction of BDNF and testosterone in the regulation of adult perineal motoneurons. J Neurobiol. 2000. 44:308–319.107. Yang LY, Verhovshek T, Sengelaub DR. Brain-derived neurotrophic factor and androgen interact in the maintenance of dendritic morphology in a sexually dimorphic rat spinal nucleus. Endocrinology. 2004. 145:161–168.108. Anderson LE, Seybold VS. Phosphorylated cAMP response element binding protein increases in neurokinin-1 receptor-immunoreactive neurons in rat spinal cord in response to formalin-induced nociception. Neurosci Lett. 2000. 283:29–32.109. Wu J, Su G, Ma L, Zhang X, Lei Y, Li J, Lin Q, Fang L. Protein kinases mediate increment of the phosphorylation of cyclic AMP-responsive element binding protein in spinal cord of rats following capsaicin injection. Mol Pain. 2005. 1:26.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Spinal Cord Stimulation for the Neuropathic Pain Caused by Traumatic Lumbosacral Plexopathy after Extensive Pelvic Fracture
- The Changes of Nitric Oxide Synthase after Spinal Cord Injury according to the AgeinRats
- Spatial and temporal expression patterns of apoptosis-related genes in rat spinal cord during normal aging
- A Comparative Study of Behavioral and immunohistological Changes after Spinal Cord Injury between Young and Adult Rats
- Age-related change of Iba-1 immunoreactivity in the adult and aged gerbil spinal cord