Anat Cell Biol.
2012 Dec;45(4):221-228. 10.5115/acb.2012.45.4.221.
Respiratory epithelial cell lines exposed to anoxia produced inflammatory mediator
- Affiliations
-
- 1Department of Pediatric Pulmonary/Allergy and Immunology, Miller Children's Hospital, University of California, Irvine, CA, USA. tchin@memorialcare.org
- 2Division of Pulmonary/Allergy and Immunology, Department of Pediatrics, Loma Linda University, Loma Linda, CA, USA.
- KMID: 2133908
- DOI: http://doi.org/10.5115/acb.2012.45.4.221
Abstract
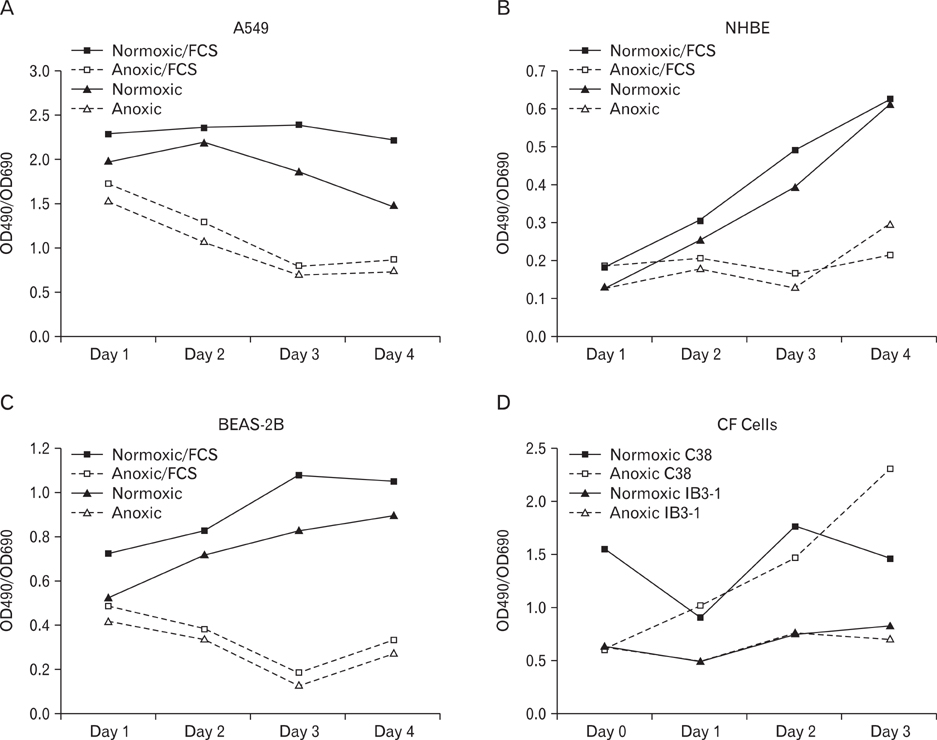
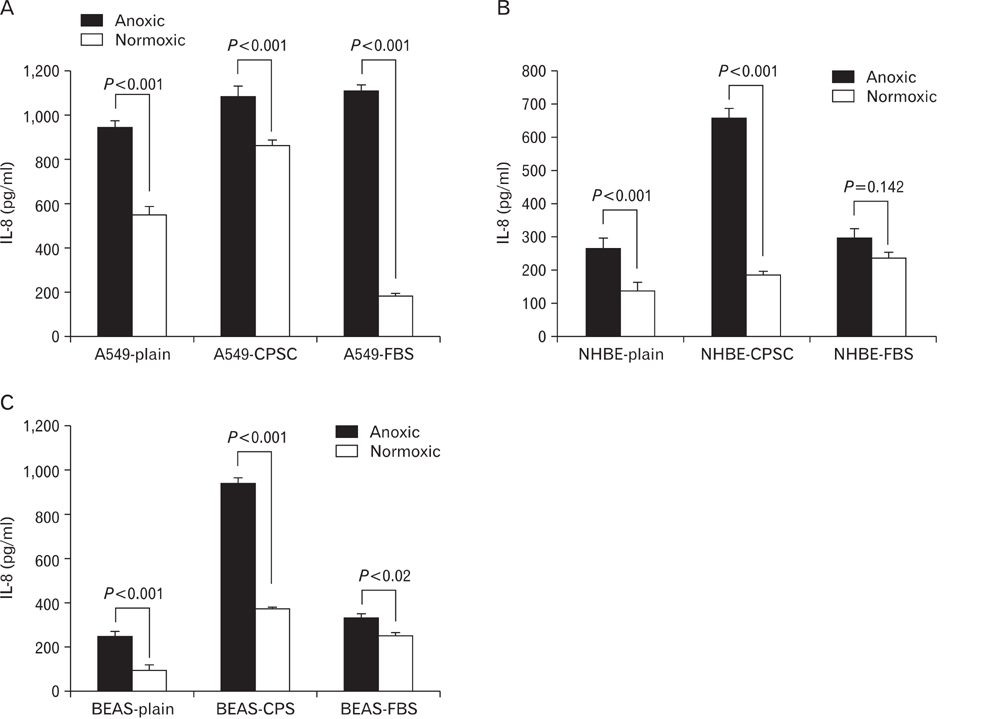
- Human epithelial cell lines were utilized to examine the effects of anoxia on cellular growth and metabolism. Three normal human epithelial cells lines (A549, NHBE, and BEAS-2B) as well as a cystic fibrosis cell line (IB3-1) and its mutation corrected cell line (C38) were grown in the presence and absence of oxygen for varying periods of time. Interleukin-8 (IL-8) levels were measured by enzyme-linked immunosorbent assay technique. Cellular metabolism and proliferation were assayed by determining mitochondrial oxidative burst activity by tetrazolium compound reduction. The viability of cells was indirectly measured by lactate dehydrogenase release. A549, NHBE, and BEAS-2B cells cultured in the absence of oxygen showed a progressive decrease in metabolic activity and cell proliferation after one to three days. There was a concomitant increase in IL-8 production. Cell lines from cystic fibrosis (CF) patients did not show a similar detrimental effect of anoxia. However, the IL-8 level was significantly increased only in IB3-1 cells exposed to anoxia after two days. Anoxia appears to affect certain airway epithelial cell lines uniquely with decreased cellular proliferation and a concomitant increased production of a cytokine with neutrophilic chemotactic activity. The increased ability of the CF cell line to respond to anoxia with increased secretion of inflammatory cytokines may contribute to the inflammatory damage seen in CF bronchial airway. This study indicates the need to use different cell lines in in vitro studies investigating the role of epithelial cells in airway inflammation and the effects of environmental influences.
Keyword
MeSH Terms
Figure
Reference
-
1. Vuichard D, Ganter MT, Schimmer RC, Suter D, Booy C, Reyes L, Pasch T, Beck-Schimmer B. Hypoxia aggravates lipopolysaccharide-induced lung injury. Clin Exp Immunol. 2005. 141:248–260.2. Madjdpour C, Jewell UR, Kneller S, Ziegler U, Schwendener R, Booy C, Kläusli L, Pasch T, Schimmer RC, Beck-Schimmer B. Decreased alveolar oxygen induces lung inflammation. Am J Physiol Lung Cell Mol Physiol. 2003. 284:L360–L367.3. Imtiyaz HZ, Williams EP, Hickey MM, Patel SA, Durham AC, Yuan LJ, Hammond R, Gimotty PA, Keith B, Simon MC. Hypoxia-inducible factor 2alpha regulates macrophage function in mouse models of acute and tumor inflammation. J Clin Invest. 2010. 120:2699–2714.4. Ghezzi P, Dinarello CA, Bianchi M, Rosandich ME, Repine JE, White CW. Hypoxia increases production of interleukin-1 and tumor necrosis factor by human mononuclear cells. Cytokine. 1991. 3:189–194.5. Signorelli S, Jennings P, Leonard MO, Pfaller W. Differential effects of hypoxic stress in alveolar epithelial cells and microvascular endothelial cells. Cell Physiol Biochem. 2010. 25:135–144.6. Jia L, Xu M, Zhen W, Shen X, Zhu Y, Wang W, Wang X. Novel anti-oxidative role of calreticulin in protecting A549 human type II alveolar epithelial cells against hypoxic injury. Am J Physiol Cell Physiol. 2008. 294:C47–C55.7. Fulcher ML, Gabriel SE, Olsen JC, Tatreau JR, Gentzsch M, Livanos E, Saavedra MT, Salmon P, Randell SH. Novel human bronchial epithelial cell lines for cystic fibrosis research. Am J Physiol Lung Cell Mol Physiol. 2009. 296:L82–L91.8. Greene CM, Ramsay H, Wells RJ, O'Neill SJ, McElvaney NG. Inhibition of Toll-like receptor 2-mediated interleukin-8 production in cystic fibrosis airway epithelial cells via the alpha7-nicotinic acetylcholine receptor. Mediators Inflamm. 2010. 2010:423241.9. Cummins EP, Taylor CT. Hypoxia-responsive transcription factors. Pflugers Arch. 2005. 450:363–371.10. Bracken CP, Whitelaw ML, Peet DJ. The hypoxia-inducible factors: key transcriptional regulators of hypoxic responses. Cell Mol Life Sci. 2003. 60:1376–1393.11. Shimoda LA, Manalo DJ, Sham JS, Semenza GL, Sylvester JT. Partial HIF-1alpha deficiency impairs pulmonary arterial myocyte electrophysiological responses to hypoxia. Am J Physiol Lung Cell Mol Physiol. 2001. 281:L202–L208.12. Leonard MO, Howell K, Madden SF, Costello CM, Higgins DG, Taylor CT, McLoughlin P. Hypoxia selectively activates the CREB family of transcription factors in the in vivo lung. Am J Respir Crit Care Med. 2008. 178:977–983.13. Vaporidi K, Tsatsanis C, Georgopoulos D, Tsichlis PN. Effects of hypoxia and hypercapnia on surfactant protein expression proliferation and apoptosis in A549 alveolar epithelial cells. Life Sci. 2005. 78:284–293.14. Voelkel NF, Vandivier RW, Tuder RM. Vascular endothelial growth factor in the lung. Am J Physiol Lung Cell Mol Physiol. 2006. 290:L209–L221.15. Hirani N, Antonicelli F, Strieter RM, Wiesener MS, Ratcliffe PJ, Haslett C, Donnelly SC. The regulation of interleukin-8 by hypoxia in human macrohages: a potential role in the pathogenesis of the acute respiratory distress syndrome (ARDS). Molecular Medicine. 2001. 7:685–697.16. Maxwell PJ, Gallagher R, Seaton A, Wilson C, Scullin P, Pettigrew J, Stratford IJ, Williams KJ, Johnston PG, Waugh DJ. HIF-1 and NF-kappaB-mediated upregulation of CXCR1 and CXCR2 expression promotes cell survival in hypoxic prostate cancer cells. Oncogene. 2007. 26:7333–7345.17. Shi Q, Le X, Abbruzzese JL, Wang B, Mujaida N, Matsushima K, Huang S, Xiong Q, Xie K. Cooperation between transcription factor AP-1 and NF-kappaB in the induction of interleukin-8 in human pancreatic adenocarcinoma cells by hypoxia. J Interferon Cytokine Res. 1999. 19:1363–1371.18. Tomlinson LA, Carpenter TC, Baker EH, Bridges JB, Weil JV. Hypoxia reduces airway epithelial sodium transport in rats. Am J Physiol. 1999. 277(5 Pt 1):L881–L886.19. Planès C, Escoubet B, Blot-Chabaud M, Friedlander G, Farman N, Clerici C. Hypoxia downregulates expression and activity of epithelial sodium channels in rat alveolar epithelial cells. Am J Respir Cell Mol Biol. 1997. 17:508–518.20. Suzuki S, Noda M, Sugita M, Ono S, Koike K, Fujimura S. Impairment of transalveolar fluid transport and lung Na(+)-K(+)-ATPase function by hypoxia in rats. J Appl Physiol. 1999. 87:962–968.21. Mairbäurl H, Mayer K, Kim KJ, Borok Z, Bärtsch P, Crandall ED. Hypoxia decreases active Na transport across primary rat alveolar epithelial cell monolayers. Am J Physiol Lung Cell Mol Physiol. 2002. 282:L659–L665.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Establishment of canine kidney cell line for canine distemper virus replication
- The Role of the Epithelial Cell in Bronchial Asthma
- The Effect of Glutathione on High Dose Cisplatin-Induced Cellular Toxicity in Non-small Cell Lung Cancer Cell Lines
- Efficiency of Chlamydia Pneumoniae Culture in the Upper Airway Epithelial Cell Lines: AMC-HN-4, AMC-HN-7, and AMC-HN-8
- Role of PI3K/Akt Pathway in the Activation of IkappaB/NF-kappaB Pathway in Lung Epithelial Cells