J Vet Sci.
2015 Dec;16(4):423-429. 10.4142/jvs.2015.16.4.423.
Elevated level of renal xanthine oxidase mRNA transcription after nephropathogenic infectious bronchitis virus infection in growing layers
- Affiliations
-
- 1Clinical Veterinary Laboratory, College of Animal Science and Technology, Jiangxi Agricultural University, Jiangxi 330029, China. Xqguo20720@aliyun.com
- KMID: 2133624
- DOI: http://doi.org/10.4142/jvs.2015.16.4.423
Abstract
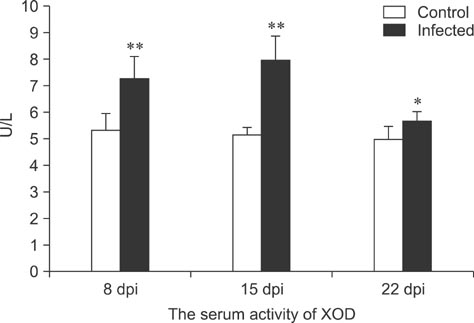
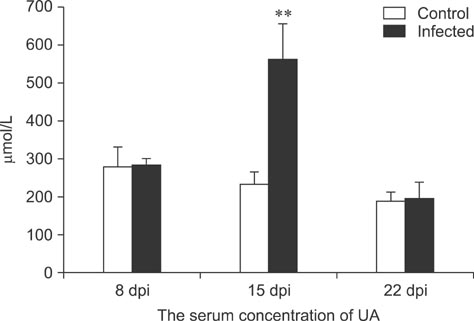
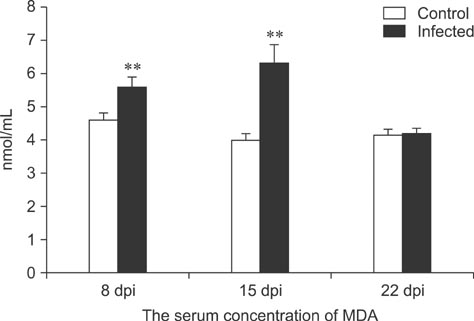
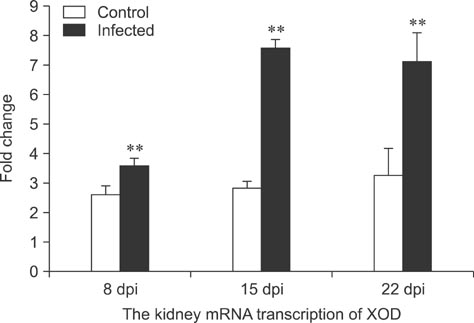
- To assess relationships between xanthine oxidase (XOD) and nephropathogenic infectious bronchitis virus (NIBV) infection, 240 growing layers (35 days old) were randomly divided into two groups (infected and control) of 120 chickens each. Each chicken in the control and infected group was intranasally inoculated with 0.2 mL sterile physiological saline and virus, respectively, after which serum antioxidant parameters and renal XOD mRNA expression in growing layers were evaluated at 8, 15 and 22 days post-inoculation (dpi). The results showed that serum glutathione peroxidase and superoxide dismutase activities in the infected group were significantly lower than in the control group at 8 and 15 dpi (p < 0.01), while serum malondialdehyde concentrations were significantly higher (p < 0.01). The serum uric acid was significantly higher than that of the control group at 15 dpi (p < 0.01). In addition, the kidney mRNA transcript level and serum activity of XOD in the infected group was significantly higher than that of the control group at 8, 15 and 22 dpi (p < 0.05). The results indicated that NIBV infection could cause the increases of renal XOD gene transcription and serum XOD activity, leading to hyperuricemia and reduction of antioxidants in the body.
Keyword
MeSH Terms
Figure
Reference
-
1. Afanador G, Roberts JR. Effect of nephropathogenic infectious bronchitis viruses on renal function in young male broiler chickens. Br Poult Sci. 1994; 35:445–456.
Article2. Agarwal A, Banerjee A, Banerjee UC. Xanthine oxidoreductase: a journey from purine metabolism to cardiovascular excitation-contraction coupling. Crit Rev Biotechnol. 2011; 31:264–280.
Article3. Al-Hakeim HK, Auda FM, Ali BM. Lack of correlation between non-labile iron parameters, total carbonyl and malondialdehyde in major thalassemia. J Clin Biochem Nutr. 2014; 55:203–206.
Article4. Asasi K, Mohammadi A, Boroomand Z, Hosseinian SA, Nazifi S. Changes of several acute phase factors in broiler chickens in response to infectious bronchitis virus infection. Poult Sci. 2013; 92:1989–1996.
Article5. Beedkar SD, Khobragade CN, Chobe SS, Dawane BS, Yemul OS. Novel thiazolo-pyrazolyl derivatives as xanthine oxidase inhibitors and free radical scavengers. Int J Biol Macromol. 2012; 50:947–956.
Article6. Celik VK, Sari I, Engin A, Gürsel Y, Aydin H, Bakir S. Determination of serum adenosine deaminase and xanthine oxidase levels in patients with crimean-congo hemorrhagic fever. Clinics (Sao Paulo). 2010; 65:697–702.
Article7. Chambers DE, Parks DA, Patterson G, Roy R, McCord JM, Yoshida S, Parmley LF, Downey JM. Xanthine oxidase as a source of free radical damage in myocardial ischemia. J Mol Cell Cardiol. 1985; 17:145–152.
Article8. Dupont GP, Huecksteadt TP, Marshall BC, Ryan US, Michael JR, Hoidal JR. Regulation of xanthine dehydrogenase and xanthine oxidase activity and gene expression in cultured rat pulmonary endothelial cells. J Clin Invest. 1992; 89:197–202.
Article9. Edwards NL. The role of hyperuricemia and gout in kidney and cardiovascular disease. Cleve Clin J Med. 2008; 75:Suppl 5. S13–S16.
Article10. Ejaz S, Kim BS, Lim CW. Gout Induced by intoxication of sodium bicarbonate in Korean native broilers. Drug Chem Toxicol. 2005; 28:245–261.
Article11. Gaba A, Dave H, Pal JK, Prajapati KS. Isolation, identification and molecular characterization of IBV variant from out break of visceral gout in commercial broilers. Vet World. 2010; 3:375–377.12. Ji J, Xie J, Chen F, Shu D, Zuo K, Xue C, Qin J, Li H, Bi Y, Ma J, Xie Q. Phylogenetic distribution and predominant genotype of the avian infectious bronchitis virus in China during 2008-2009. Virol J. 2011; 8:184.
Article13. Kang DH, Nakagawa T. Uric acid and chronic renal disease: Possible implication of hyperuricemia on progression of renal disease. Semin Nephrol. 2005; 25:43–49.
Article14. Lee CW, Brown C, Hilt DA, Jackwood MW. Nephropathogenesis of chickens experimentally infected with various strains of infectious bronchitis virus. J Vet Med Sci. 2004; 66:835–840.
Article15. Liu MD, Luo P, Wang ZJ, Fei Z. Changes of serum Tau, GFAP, TNF-α and malonaldehyde after blast-related traumatic brain injury. Chin J Traumatol. 2014; 17:317–322.16. Many A, Hubel CA, Roberts JM. Hyperuricemia and xanthine oxidase in preeclampsia, revisited. Am J Obstet Gynecol. 1996; 174:288–291.
Article17. Naguib YM, Azmy RM, Samaka RM, Salem MF. Pleurotus ostreatus opposes mitochondrial dysfunction and oxidative stress in acetaminophen-induced hepato-renal injury. BMC Complement Altern Med. 2014; 14:494.
Article18. Nath KA, Norby SM. Reactive oxygen species and acute renal failure. Am J Med. 2000; 109:665–678.
Article19. Nemzer B, Chang T, Xie Z, Pietrzkowski Z, Reyes T, Ou B. Decrease of free radical concentrations in humans following consumption of a high antioxidant capacity natural product. Food Sci Nutr. 2014; 2:647–654.
Article20. Okamoto K, Kusano T, Nishino T. Chemical nature and reaction mechanisms of the molybdenum cofactor of xanthine oxidoreductase. Curr Pharm Des. 2013; 19:2606–2614.
Article21. Okino CH, dos Santos IL, Fernando FS, Alessi AC, Wang X, Montassier HJ. Inflammatory and cell-mediated immune responses in the respiratory tract of chickens to infection with avian infectious bronchitis virus. Viral Immunol. 2014; 27:383–391.
Article22. Peralta C, Bulbena O, Xaus C, Prats N, Cutrin JC, Poli G, Gelpi E, Roselló-Catafau J. Ischemic preconditioning: a defense mechanism against the reactive oxygen species generated after hepatic ischemia reperfusion. Transplantation. 2002; 73:1203–1211.
Article23. Pfeffer KD, Huecksteadt TP, Hoidal JR. Xanthine dehydrogenase and xanthine oxidase activity and gene expression in renal epithelial cells. Cytokine and steroid regulation. J Immunol. 1994; 153:1789–1797.24. Poss WB, Huecksteadt TP, Panus PC, Freeman BA, Hoidal JR. Regulation of xanthine dehydrogenase and xanthine oxidase activity by hypoxia. Am J Physiol. 1996; 270:L941–L946.
Article25. Qian DH, Zhu GJ, Wu LZ, Hua GX. Isolation and characterization of a coronavirus from pigeons with pancreatitis. Am J Vet Res. 2006; 67:1575–1579.
Article26. Reed LJ, Muench H. A simple method of estimating fifty per cent endpoints. Am J Epidemiol. 1938; 27:493–497.27. Reyazuddin M, Azmi SA, Islam N, Rizvi A. Oxidative stress and level of antioxidant enzymes in drug-naive schizophrenics. Indian J Psychiatry. 2014; 56:344–349.
Article28. Sato A, Nishino T, Noda K, Amaya Y, Nishino T. The structure of chicken liver xanthine dehydrogenase. cDNA cloning and the domain structure. J Biol Chem. 1995; 270:2818–2826.29. Seifried HE, Anderson DE, Fisher EI, Milner JA. A review of the interaction among dietary antioxidants and reactive oxygen species. J Nutr Biochem. 2007; 18:567–579.
Article30. Singh N, Ghosh RC, Singh A. Prevalence and haemato-biochemical studies on naturally occurring gout in Chhattisgarh. Adv Anim Vet Sci. 2013; 1:3S. 9–11.31. Taş S, Sarandöl E, Dirican M. Vitamin B6 supplementation improves oxidative stress and enhances serum paraoxonase/arylesterase activities in streptozotocin-induced diabetic rats. ScientificWorldJournal. 2014; 2014:351598.
Article32. Vorbach C, Harrison R, Capecchi MR. Xanthine oxidoreductase is central to the evolution and function of the innate immune system. Trends Immunol. 2003; 24:512–517.
Article33. Wideman RF Jr, Cowen BS. Effect of dietary acidification on kidney damage induced in immature chickens by excess calcium and infectious bronchitis virus. Poult Sci. 1987; 66:626–633.
Article34. Xie Q, Ji J, Xie J, Chen F, Cai M, Sun B, Xue C, Ma J, Bi Y. Epidemiology and immunoprotection of nephropathogenic avian infectious bronchitis virus in southern China. Virol J. 2011; 8:484.
Article35. Ziegler AF, Ladman BS, Dunn PA, Schneider A, Davison S, Miller PG, Lu H, Weinstock D, Salem M, Eckroade RJ, Gelb J Jr. Nephropathogenic infectious bronchitis in Pennsylvania chickens 1997-2000. Avian Dis. 2002; 46:847–858.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Pathogenicity and antigenicity of a new variant of Korean nephropathogenic infectious bronchitis virus
- The potential renoprotection of xanthine oxidase inhibitors: Febuxostat versus allopurinol
- Analysis of Relationship Between Spermatozoa Ability and Reactive Oxygen Species in Porcine: I. Sperm Preincubation by Xanthine and Xanthine Oxidase
- Development of an attenuated vaccine strain from a korean respiratory type infectious bronchitis virus
- A Case of Allopurinol Hypersensitivity Syndrome