J Vet Sci.
2015 Dec;16(4):389-396. 10.4142/jvs.2015.16.4.389.
Anti-adipogenic effect of Artemisia annua in diet-induced-obesity mice model
- Affiliations
-
- 1Department of Biomedical Laboratory Science, College of Medical Sciences, Soonchunhyang University, Asan 31538, Korea. admiral96@sch.ac.kr
- 2Department of Medical Biotechnology, College of Medical Sciences, Soonchunhyang University, Asan 31538, Korea.
- 3Department of Life Science and Biotechnology, College of Natural Sciences, Soonchunhyang University, Asan 31538, Korea.
- 4Genomic Informatic Center, Han-kyong National University, Anseong 17579, Korea.
- 5Department of Biotechnology, Hoseo University, Asan 31499, Korea.
- KMID: 2133620
- DOI: http://doi.org/10.4142/jvs.2015.16.4.389
Abstract
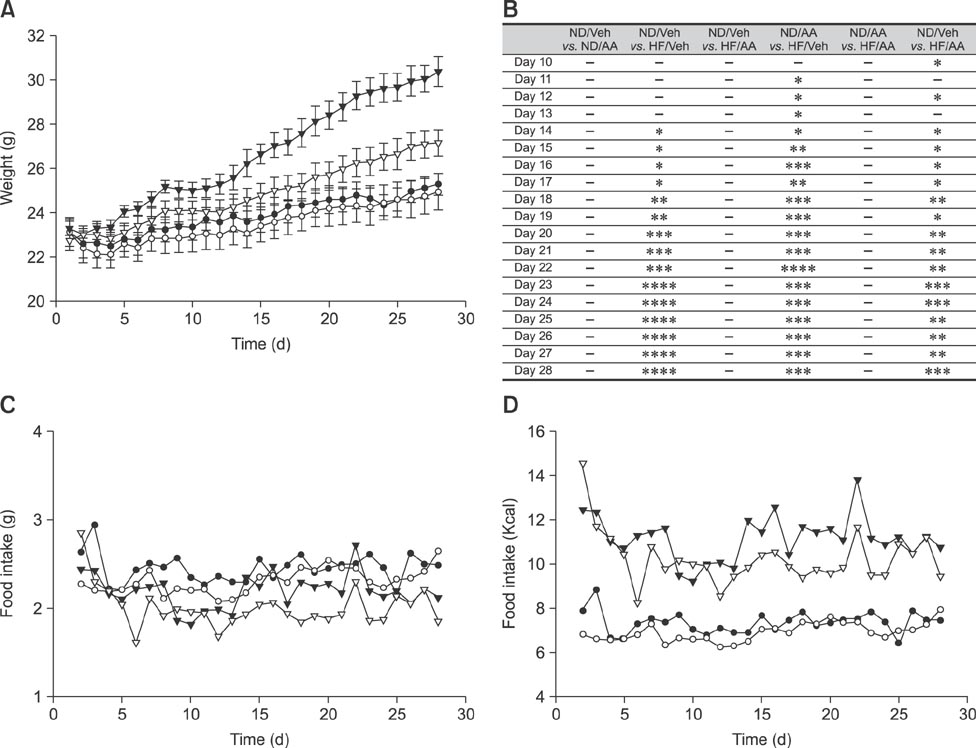
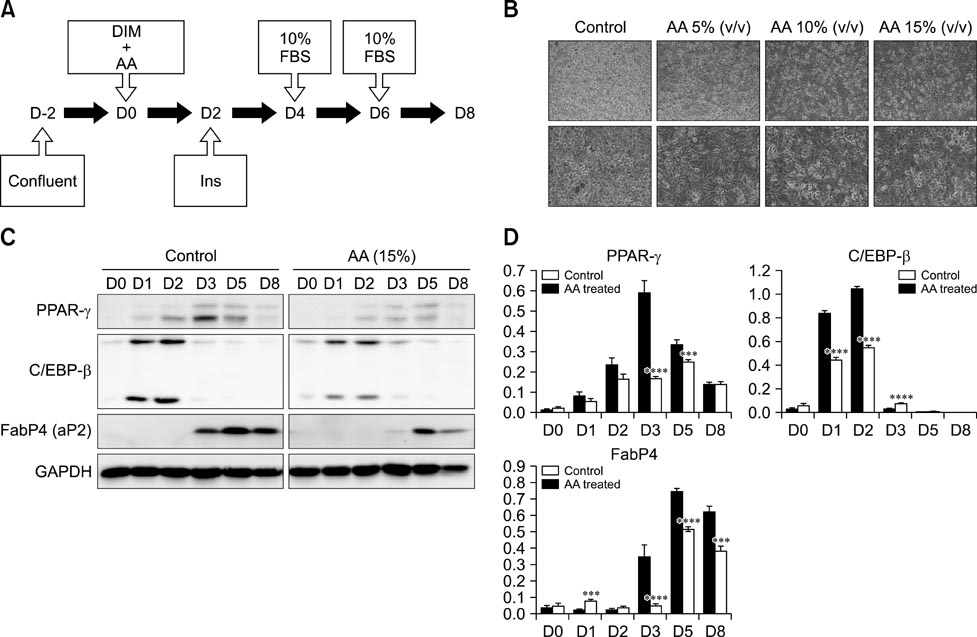
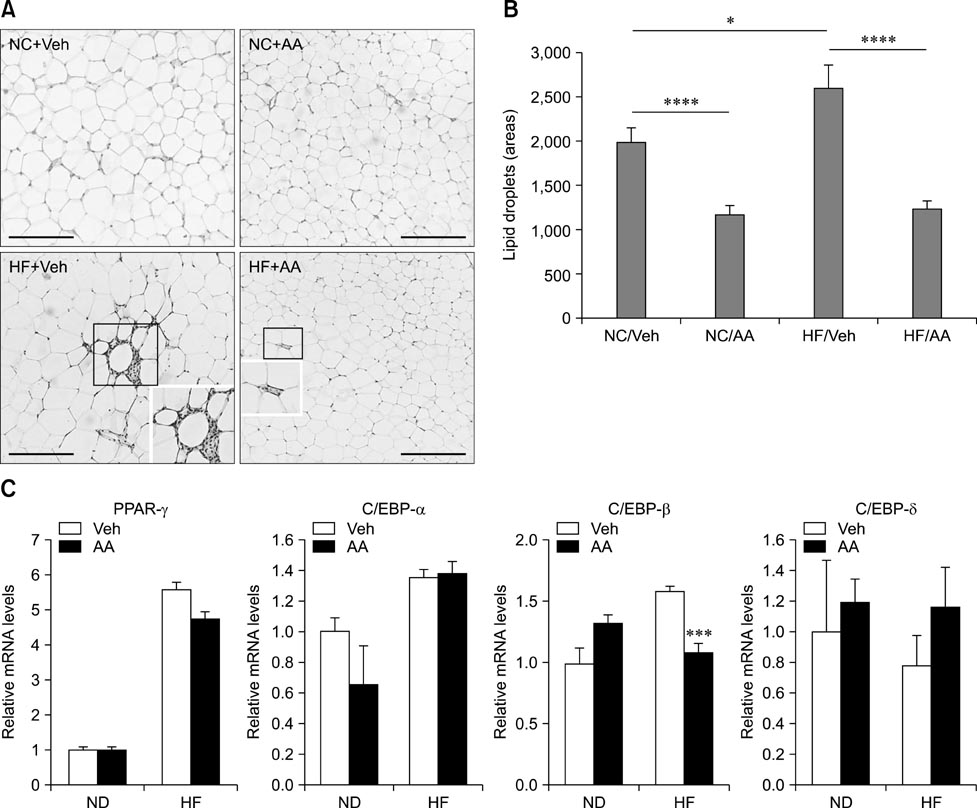
- Obesity has increased continuously in western countries during the last several decades and recently become a problem in developing countries. Currently, anti-obesity drugs originating from natural products are being investigated for their potential to overcome adverse effects associated with chemical drugs. Artemisinic acid, which was isolated from the well-known anti-malaria herb Artemisia annua (AA) L., was recently shown to possess anti-adipogenic effects in vitro. However, the anti-adipogenic effects of AA in animal models have not yet been investigated. Therefore, we conducted daily oral administration with AA water extract in a diet-induced obesity animal model and treated 3T3-L1 cells with AA to confirm the anti-adipogenic effects in the related protein expressions. We then evaluated the physiology, adipose tissue histology and mRNA expressions of many related genes. Inhibition of adipogenesis by the AA water extract was observed in vitro. In the animal model, weight gain was significantly lower in the AA treated group, but there were no changes in food intake volume or calories. Reductions in lipid droplet size and mRNA expression associated with adipogenesis were also observed in animal epididymal fat. This study is the first to report that AA has an anti-obese effects in vivo.
MeSH Terms
Figure
Cited by 1 articles
-
Neuronal maturation in the hippocampal dentate gyrus via chronic oral administration of Artemisa annua extract is independent of cyclooxygenase 2 signaling pathway in diet-induced obesity mouse model
Hye Kyung Baek, Pan Soo Kim, Ji Ae Song, Dong-Hwa Choi, Do Eun Kim, Seung Il Oh, Sang-Kyu Park, Sung-Jo Kim, Ki-Duk Song, In Koo Hwang, Hyung Seok Seo, Sun Shin Yi
J Vet Sci. 2017;18(2):119-127. doi: 10.4142/jvs.2017.18.2.119.
Reference
-
1. Aral LA, Pinar L, Göktaş G, Deveden EY, Erdoğan D. Comparison of hippocampal interleukin-6 immunoreactivity after exhaustive exercise in both exercise-trained and untrained rats. Turk J Med Sci. 2014; 44:560–568.
Article2. Bag S, Ramaiah S, Anbarasu A. fabp4 is central to eight obesity associated genes: a functional gene network-based polymorphic study. J Theor Biol. 2015; 364:344–354.
Article3. Bai Y, Sun Q. Macrophage recruitment in obese adipose tissue. Obes Rev. 2015; 16:127–136.
Article4. Ballinger A. Orlistat in the treatment of obesity. Expert Opin Pharmacother. 2000; 1:841–847.
Article5. Bello NT, Walters AL, Verpeut JL, Cunha PP. High-fat diet-induced alterations in the feeding suppression of low-dose nisoxetine, a selective norepinephrine reuptake inhibitor. J Obes. 2013; 2013:457047.
Article6. Choi S, Blake V, Cole S, Fernstrom JD. Effects of chronic fenfluramine administration on hypothalamic neuropeptide mRNA expression. Brain Res. 2006; 1087:83–86.
Article7. Choi S, Jonak EM, Simpson L, Patil V, Fernstrom JD. Intermittent, chronic fenfluramine administration to rats repeatedly suppresses food intake despite substantial brain serotonin reductions. Brain Res. 2002; 928:30–39.
Article8. Comerma-Steffensen S, Grann M, Andersen CU, Rungby J, Simonsen U. Cardiovascular effects of current and future anti-obesity drugs. Curr Vasc Pharmacol. 2014; 12:493–504.
Article9. Coskun H, Summerfield TLS, Kniss DA, Friedman A. Mathematical modeling of preadipocyte fate determination. J Theor Biol. 2010; 265:87–94.
Article10. Exley MA, Hand L, O'Shea D, Lynch L. Interplay between the immune system and adipose tissue in obesity. J Endocrinol. 2014; 223:R41–R48.
Article11. Fantuzzi G. Adipose tissue, adipokines, and inflammation. J Allergy Clin Immunol. 2005; 115:911–919.
Article12. Frühbeck G. Overview of adipose tissue and its role in obesity and metabolic disorders. Methods Mol Biol. 2008; 456:1–22.
Article13. Fu M, Sun T, Bookout AL, Downes M, Yu RT, Evans RM, Mangelsdorf DJ. A nuclear receptor atlas: 3T3-L1 adipogenesis. Mol Endocrinol. 2005; 19:2437–2450.
Article14. Garin-Shkolnik T, Rudich A, Hotamisligil GS, Rubinstein M. FABP4 attenuates PPARγ and adipogenesis and is inversely correlated with PPARγ in adipose tissues. Diabetes. 2014; 63:900–911.
Article15. Hsueh WA, Law R. The central role of fat and effect of peroxisome proliferator-activated receptor-γ on progression of insulin resistance and cardiovascular disease. Am J Cardiol. 2003; 92:Suppl. 3J–9J.
Article16. Jackson HC, Bearham MC, Hutchins LJ, Mazurkiewicz SE, Needham AM, Heal DJ. Investigation of the mechanisms underlying the hypophagic effects of the 5-HT and noradrenaline reuptake inhibitor, sibutramine, in the rat. Br J Pharmacol. 1997; 121:1613–1618.
Article17. Jeong HW, Lee JW, Kim WS, Choe SS, Kim KH, Park HS, Shin HJ, Lee GY, Shin D, Lee H, Lee JH, Choi EB, Lee HK, Chung H, Park SB, Park KS, Kim HS, Ro S, Kim JB. A newly identified CG301269 improves lipid and glucose metabolism without body weight gain through activation of peroxisome proliferator-activated receptor α and γ. Diabetes. 2011; 60:496–506.
Article18. Kim SK, Kim HJ, Hur KY, Choi SH, Ahn CW, Lim SK, Kim KR, Lee HC, Huh KB, Cha BS. Visceral fat th ickness measured by ultrasonography can estimate not only visceral obesity but also risks of cardiovascular and metabolic diseases. Am J Clin Nutr. 2004; 79:593–599.
Article19. Knudsmark Jessing K, Duke SO, Cedergreeen N. Potential ecological roles of artemisinin produced by Artemisia annua L. J Chem Ecol. 2014; 40:100–117.
Article20. Lai CS, Tsai ML, Badmaev V, Jimenez M, Ho CT, Pan MH. Xanthigen suppresses preadipocyte differentiation and adipogenesis through down-regulation of PPARγ and C/EBPs and modulation of SIRT-1, AMPK, and FoxO pathways. J Agric Food Chem. 2012; 60:1094–1101.
Article21. Lee H, Kang R, Hahn Y, Yang Y, Kim SS, Cho SH, Chung SI, Yoon Y. Antiobesity effect of baicalin involves the modulations of proadipogenic and antiadipogenic regulators of the adipogenesis pathway. Phytother Res. 2009; 23:1615–1623.
Article22. Lee J, Kim MH, Lee JH, Jung E, Yoo ES, Park D. Artemisinic acid is a regulator of adipocyte differentiation and C/EBP δ expression. J Cell Biochem. 2012; 113:2488–2499.
Article23. Lee MR. Plants against malaria, part 2: Artemisia annua (Qinghaosu or the sweet wormwood). J R Coll Physicians Edinb. 2002; 32:300–305.24. McDonald MK, Tian Y, Qureshi RA, Gormley M, Ertel A, Gao R, Aradillas Lopez E, Alexander GM, Sacan A, Fortina P, Ajit SK. Functional significance of macrophage-derived exosomes in inflammation and pain. Pain. 2014; 155:1527–1539.
Article25. Peterson HR, Rothschild M, Weinberg CR, Fell RD, McLeish KR, Pfeifer MA. Body fat and the activity of the autonomic nervous system. N Engl J Med. 1988; 318:1077–1083.
Article26. Pikkel YY. Acute bilateral glaucoma and panuveitis as a side effect of topiramate for weight loss treatment. BMJ Case Rep. 2014; 2014:bcr2014203787.
Article27. Qi S, Zhou D. Lotus seed epicarp extract as potential antioxidant and anti-obesity additive in Chinese Cantonese sausage. Meat Sci. 2013; 93:257–262.
Article28. Rethorst CD, Bernstein I, Trivedi MH. Inflammation, obesity, and metabolic syndrome in depression: analysis of the 2009-2010 National Health and Nutrition Examination Survey (NHANES). J Clin Psychiatry. 2014; 75:e1428–e1432.29. RITAM Artemisia annua Task Force. Falquet J, Ferreira JF, Gilbert B, Hsu E, de Magalhães PM, Plaizier-Vercammen J, Sharma VP, Wright CW, Yaode W. Artemisia annua as a herbal tea for malaria. Afr J Tradit Complement Altern Med. 2006; 4:121–123.30. Scott B, Lew J, Clandinin MT, Cinader B. Dietary fat influences electric membrane properties of neurons in cell culture. Cell Mol Neurobiol. 1989; 9:105–113.
Article31. Siebenhofer A, Jeitler K, Horvath K, Berghold A, Siering U, Semlitsch T. Long-term effects of weight-reducing drugs in hypertensive patients. Cochrane Database Syst Rev. 2013; 3:CD007654.
Article32. Tan S, Gao B, Tao Y, Guo J, Su ZQ. Antiobese effects of capsaicin-chitosan microsphere (CCMS) in obese rats induced by high fat diet. J Agric Food Chem. 2014; 62:1866–1874.
Article33. Taupin V, Gogusev J, Descamps-Latscha B, Zavala F. Modulation of tumor necrosis factor-α, interleukin-1β, interleukin-6, interleukin-8, and granulocyte/macrophage colony-stimulating factor expression in human monocytes by an endogenous anxiogenic benzodiazepine ligand, triakontatetraneuropeptide: evidence for a role of prostaglandins. Mol Pharmacol. 1993; 43:64–69.34. Tiele OW. Fat, hypertension and arteriosclerosis in the experimental animal. Hippokrates. 1974; 45:512.35. Torkildsen Ø, Løken-Amsrud KI, Wergeland S, Myhr KM, Holmøy T. Fat-soluble vitamins as disease modulators in multiple sclerosis. Acta Neurol Scand Suppl. 2013; s196:16–23.
Article36. Vereyken EJF, Bajova H, Chow S, De Graan PNE, Gruol DL. Chronic interleukin-6 alters the level of synaptic proteins in hippocampus in culture and in vivo. Eur J Neurosci. 2007; 25:3605–3616.
Article37. Wu B, Hu K, Li S, Zhu J, Gu L, Shen H, Hambly BD, Bao S, Di W. Dihydroartiminisin inhibits the growth and metastasis of epithelial ovarian cancer. Oncol Rep. 2012; 27:101–108.
Article38. Xing Z, Jordana M, Kirpalani H, Driscoll KE, Schall TJ, Gauldie J. Cytokine expression by neutrophils and macrophages in vivo: endotoxin induces tumor necrosis factor-alpha, macrophage inflammatory protein-2, interleukin-1 beta, and interleukin-6 but not RANTES or transforming growth factor-beta 1 mRNA expression in acute lung inflammation. Am J Respir Cell Mol Biol. 1994; 10:148–153.
Article39. Yang HN, Park JS, Woo DG, Jeon SY, Do HJ, Lim HY, Kim JH, Park KH. C/EBP-α and C/EBP-β-mediated adipogenesis of human mesenchymal stem cells (hMSCs) using PLGA nanoparticles complexed with poly (ethyleneimmine). Biomaterials. 2011; 32:5924–5933.
Article40. Yoshioka S, Uemura K, Tamaya N, Tamagawa T, Miura H, Iguchi A, Nakamura J, Hotta N. Dietary fat-induced increase in blood pressure and insulin resistance in rats. J Hypertens. 2000; 18:1857–1864.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- What is In-Jin-Sook? Artemisia capillaris, Artemisia iwayomogi, and Artemisia annua
- Anti-Obesity Effects of Artemisia annua Extract in Zucker Fatty Rats and High-Fat Diet Sprague Dawley Rats through Upregulation of Uncoupling Protein 1
- Seizure Induction by Artemisia Annua in an Epilepsy Patient Taking Levetiracetam
- Neuronal maturation in the hippocampal dentate gyrus via chronic oral administration of Artemisa annua extract is independent of cyclooxygenase 2 signaling pathway in diet-induced obesity mouse model
- Artemisia annua extract ameliorates high-fat diet-induced fatty liver by activating AMPK