Korean J Ophthalmol.
2014 Jun;28(3):275-277. 10.3341/kjo.2014.28.3.275.
Suspected Bacterial Endophthalmitis Following Sustained-release Dexamethasone Intravitreal Implant: A Case Report
- Affiliations
-
- 1Ophthalmology Department, Ankara Ataturk Training and Research Hospital, Ankara, Turkey. mcllarkn@yahoo.com
- 2Department of Ophthalmology, Yildirim Beyazit University, Ankara, Turkey.
- KMID: 2133329
- DOI: http://doi.org/10.3341/kjo.2014.28.3.275
Abstract
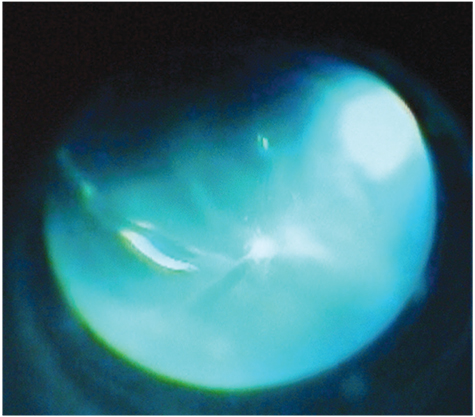
- A 58-year-old man admitted to our opthalmology department with the complaint of branch retinal vein occlusion. He was treated with intravitreal Ozurdex in the right eye. Two days after the injection, the patient presented with ocular pain and the visual acuity was hand movement. A diagnosis of endophthalmitis was made. We performed emergent pars plana vitrectomy (PPV) and the implant was removed from the vitreous cavity using a retinal forceps. A combination of vancomycin 1.0 mg and amikacin 0.4 mg was injected intravitreally. However, because of the blurring in the vitreus one week after the procedure, phacoemulsification and a repeat PPV was performed. Five days after the last procedure the signs and symptoms of endophthalmitis were resolved. Our case demonstrated that endophthalmitis could develop after intravitreal implantation of Ozurdex. Surgical removal of the implant and immediate vitrectomy seems to be a useful treatment option in these cases.
MeSH Terms
-
Device Removal/methods
Dexamethasone/administration & dosage/*adverse effects
Diagnosis, Differential
Drug Implants/*adverse effects
Endophthalmitis/diagnosis/*etiology/surgery
Eye Infections, Bacterial/diagnosis/*etiology/surgery
Glucocorticoids/administration & dosage/adverse effects
Humans
Intravitreal Injections/adverse effects
Male
Middle Aged
Retinal Vein Occlusion/diagnosis/*drug therapy
Vitrectomy
Dexamethasone
Drug Implants
Glucocorticoids
Figure
Reference
-
1. Schmitz K, Maier M, Clemens CR, et al. The ZERO study. Reliability and safety of intravitreal Ozurdex injections. Ophthalmologe. 2014; 111:44–52.2. Haller JA, Bandello F, Belfort R Jr, et al. Dexamethasone intravitreal implant in patients with macular edema related to branch or central retinal vein occlusion twelve-month study results. Ophthalmology. 2011; 118:2453–2460.3. Marchino T, Vela JI, Bassaganyas F, et al. Acute-onset endophthalmitis caused by alloiococcus otitidis following a dexamethasone intravitreal implant. Case Rep Ophthalmol. 2013; 4:37–41.4. London NJ, Chiang A, Haller JA. The dexamethasone drug delivery system: indications and evidence. Adv Ther. 2011; 28:351–366.5. Endophthalmitis Vitrectomy Study Group. Results of the Endophthalmitis Vitrectomy Study. A randomized trial of immediate vitrectomy and of intravenous antibiotics for the treatment of postoperative bacterial endophthalmitis. Arch Ophthalmol. 1995; 113:1479–1496.6. Kuhn F, Gini G. Ten years after... are findings of the Endophthalmitis Vitrectomy Study still relevant today? Graefes Arch Clin Exp Ophthalmol. 2005; 243:1197–1199.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Acute Endophthalmitis Following a Dexamethasone Intravitreal Implant
- A Case of Retinal Hemorrhage Following a Dexamethasone Intravitreal Implant
- A Case of Postoperative Bacterial Endophthalmitis after Successful Treatment of Suspected Iatrogenic Fungal Endophthalmitis
- A Case of Pseudomonas Aeruginosa Endophthalmitis Treated with Intravitreal Ceftazidime Injection
- A Case of Repeated Intravitreal Dexamethasone Implantation for Treatment of Macular Edema after Scleral Fixation of Intraocular Lens