Anat Cell Biol.
2015 Dec;48(4):235-243. 10.5115/acb.2015.48.4.235.
Protective effects of kaempferol against cardiac sinus node dysfunction via CaMKII deoxidization
- Affiliations
-
- 1Department of Medicine, Ewha Womans University School of Medicine, Seoul, Korea. ms@ewha.ac.kr
- KMID: 2133262
- DOI: http://doi.org/10.5115/acb.2015.48.4.235
Abstract
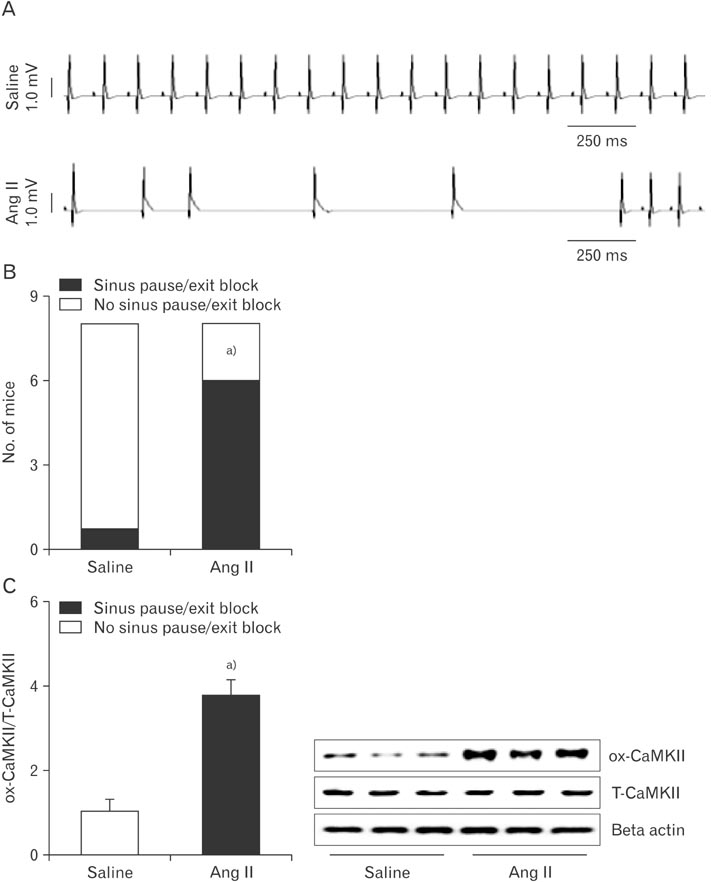
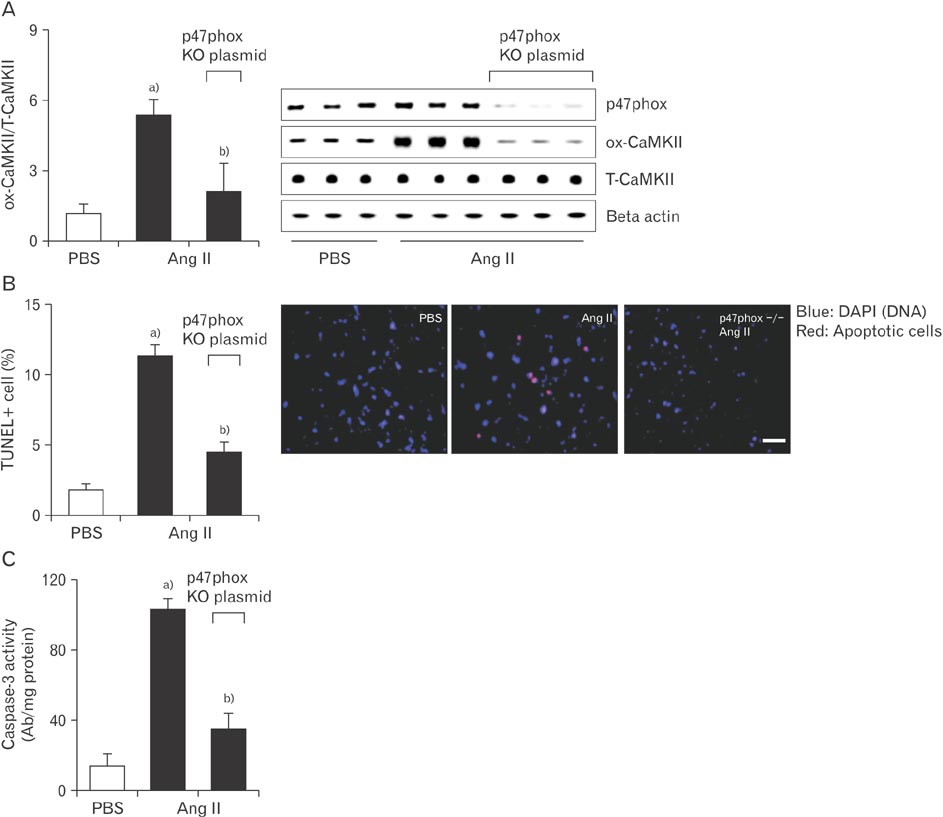
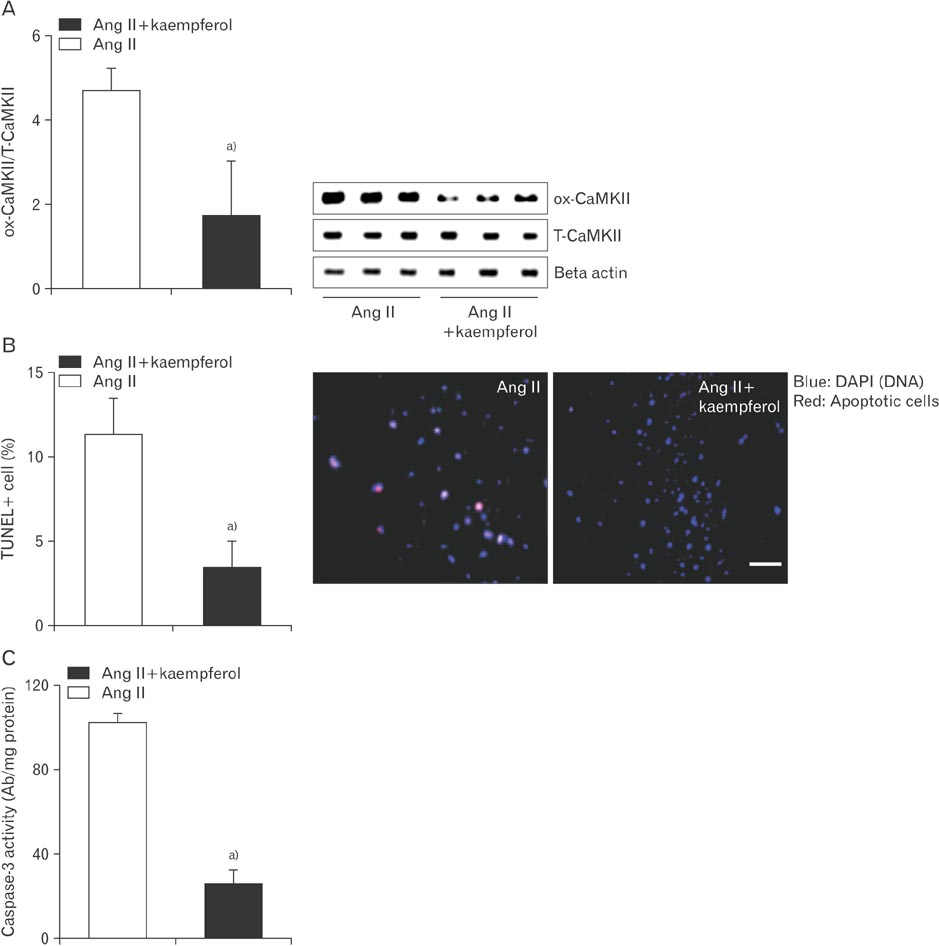
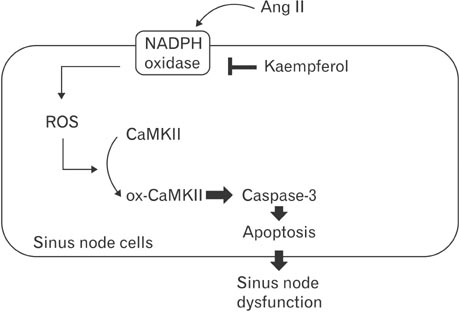
- Kaempferol exerts cardioprotective actions through incompletely understood mechanisms. This study investigated the molecular mechanisms underlying the cardioprotective effects of kaempferol in sinus node dysfunction (SND) heart. Here, we demonstrate that angiotensin II (Ang II) infusion causes SND through oxidized calmodulin kinase II (CaMKII). In contrast to this, kaempferol protects sinus node against Ang II-induced SND. Ang II evoked apoptosis with caspase-3 activation in sinus nodal cells. However, kaempferol lowered the CaMKII oxidization and the sinus nodal cell death. To block the CaMKII oxidization, gene of p47phox, a cytosolic subunit of NADPH oxidase, was deleted using Cas9 KO plasmid. In the absence of p47phox, sinus nodal cells were highly resistance to Ang II-induced apoptosis, suggesting that oxidized-CaMKII contributed to sinus nodal cell death. In Langendorff heart from Ang II infused mice, kaempferol preserved normal impulse formation at right atrium. These data suggested that kaempferol protects sinus node via inhibition of CaMKII oxidization and may be useful for preventing SND in high risk patients.
Keyword
MeSH Terms
-
Angiotensin II
Animals
Apoptosis
Calcium-Calmodulin-Dependent Protein Kinase Type 2*
Calcium-Calmodulin-Dependent Protein Kinases
Caspase 3
Cell Death
Cytosol
Heart
Heart Atria
Humans
Mice
NADPH Oxidase
Plasmids
Sick Sinus Syndrome*
Sinoatrial Node*
Angiotensin II
Calcium-Calmodulin-Dependent Protein Kinase Type 2
Calcium-Calmodulin-Dependent Protein Kinases
Caspase 3
NADPH Oxidase
Figure
Reference
-
1. Liu J, Xin L, Benson VL, Allen DG, Ju YK. Store-operated calcium entry and the localization of STIM1 and Orai1 proteins in isolated mouse sinoatrial node cells. Front Physiol. 2015; 6:69.2. Milanesi R, Bucchi A, Baruscotti M. The genetic basis for inherited forms of sinoatrial dysfunction and atrioventricular node dysfunction. J Interv Card Electrophysiol. 2015; 43:121–134.3. Swulius MT, Waxham MN. Ca(2+)/calmodulin-dependent protein kinases. Cell Mol Life Sci. 2008; 65:2637–2657.4. Yang LZ, Kockskämper J, Khan S, Suarez J, Walther S, Doleschal B, Unterer G, Khafaga M, Mächler H, Heinzel FR, Dillmann WH, Pieske B, Spiess J. cAMP- and Ca(2)(+) /calmodulin-dependent protein kinases mediate inotropic, lusitropic and arrhythmogenic effects of urocortin 2 in mouse ventricular myocytes. Br J Pharmacol. 2011; 162:544–556.5. Wang Y, Cui H, Wang W, Zhao B, Lai J. The region-specific activation of Ca2+/calmodulin dependent protein kinase II and extracellular signal-regulated kinases in hippocampus following chronic alcohol exposure. Brain Res Bull. 2012; 89:191–196.6. Huynh QK, Pagratis N. Kinetic mechanisms of Ca++/calmodulin dependent protein kinases. Arch Biochem Biophys. 2011; 506:130–136.7. Erickson JR, Joiner ML, Guan X, Kutschke W, Yang J, Oddis CV, Bartlett RK, Lowe JS, O'Donnell SE, Aykin-Burns N, Zimmerman MC, Zimmerman K, Ham AJ, Weiss RM, Spitz DR, Shea MA, Colbran RJ, Mohler PJ, Anderson ME. A dynamic pathway for calcium-independent activation of CaMKII by methionine oxidation. Cell. 2008; 133:462–474.8. Santalla M, Valverde CA, Harnichar E, Lacunza E, Aguilar-Fuentes J, Mattiazzi A, Ferrero P. Aging and CaMKII alter intracellular Ca2+ transients and heart rhythm in Drosophila melanogaster. PLoS One. 2014; 9:e101871.9. Di Carlo MN, Said M, Ling H, Valverde CA, De Giusti VC, Sommese L, Palomeque J, Aiello EA, Skapura DG, Rinaldi G, Respress JL, Brown JH, Wehrens XH, Salas MA, Mattiazzi A. CaMKII-dependent phosphorylation of cardiac ryanodine receptors regulates cell death in cardiac ischemia/reperfusion injury. J Mol Cell Cardiol. 2014; 74:274–283.10. Szobi A, Rajtik T, Carnicka S, Ravingerova T, Adameova A. Mitigation of postischemic cardiac contractile dysfunction by CaMKII inhibition: effects on programmed necrotic and apoptotic cell death. Mol Cell Biochem. 2014; 388:269–276.11. Zhao HR, Jiang T, Tian YY, Gao Q, Li Z, Pan Y, Wu L, Lu J, Zhang YD. Angiotensin II triggers apoptosis via enhancement of NADPH oxidase-dependent oxidative stress in a dopaminergic neuronal cell line. Neurochem Res. 2015; 40:854–863.12. Carney EF. Hypertension: vascular type 1A angiotensin II receptors regulate renal blood flow and natriuresis. Nat Rev Nephrol. 2015; 11:318.13. Rincon J, Correia D, Arcaya JL, Finol E, Fernández A, Pérez M, Yaguas K, Talavera E, Chávez M, Summer R, Romero F. Role of angiotensin II type 1 receptor on renal NAD(P)H oxidase, oxidative stress and inflammation in nitric oxide inhibition induced-hypertension. Life Sci. 2015; 124:81–90.14. Swaminathan PD, Purohit A, Soni S, Voigt N, Singh MV, Glukhov AV, Gao Z, He BJ, Luczak ED, Joiner ML, Kutschke W, Yang J, Donahue JK, Weiss RM, Grumbach IM, Ogawa M, Chen PS, Efimov I, Dobrev D, Mohler PJ, Hund TJ, Anderson ME. Oxidized CaMKII causes cardiac sinus node dysfunction in mice. J Clin Invest. 2011; 121:3277–3288.15. Zhang R, Khoo MS, Wu Y, Yang Y, Grueter CE, Ni G, Price EE Jr, Thiel W, Guatimosim S, Song LS, Madu EC, Shah AN, Vishnivetskaya TA, Atkinson JB, Gurevich VV, Salama G, Lederer WJ, Colbran RJ, Anderson ME. Calmodulin kinase II inhibition protects against structural heart disease. Nat Med. 2005; 11:409–417.16. Luo M, Guan X, Luczak ED, Lang D, Kutschke W, Gao Z, Yang J, Glynn P, Sossalla S, Swaminathan PD, Weiss RM, Yang B, Rokita AG, Maier LS, Efimov IR, Hund TJ, Anderson ME. Diabetes increases mortality after myocardial infarction by oxidizing CaMKII. J Clin Invest. 2013; 123:1262–1274.17. McCullough ML, Peterson JJ, Patel R, Jacques PF, Shah R, Dwyer JT. Flavonoid intake and cardiovascular disease mortality in a prospective cohort of US adults. Am J Clin Nutr. 2012; 95:454–464.18. Esmaillzadeh A, Azadbakht L. Dietary flavonoid intake and cardiovascular mortality. Br J Nutr. 2008; 100:695–697.19. Chong MF, George TW, Alimbetov D, Jin Y, Weech M, Macready AL, Spencer JP, Kennedy OB, Minihane AM, Gordon MH, Lovegrove JA. Impact of the quantity and flavonoid content of fruits and vegetables on markers of intake in adults with an increased risk of cardiovascular disease: the FLAVURS trial. Eur J Nutr. 2013; 52:361–378.20. Barbosa E, Calzada F, Campos R. In vivo antigiardial activity of three flavonoids isolated of some medicinal plants used in Mexican traditional medicine for the treatment of diarrhea. J Ethnopharmacol. 2007; 109:552–554.21. Xiao HB, Jun F, Lu XY, Chen XJ, Chao T, Sun ZL. Protective effects of kaempferol against endothelial damage by an improvement in nitric oxide production and a decrease in asymmetric dimethylarginine level. Eur J Pharmacol. 2009; 616:213–222.22. Wang SB, Jang JY, Chae YH, Min JH, Baek JY, Kim M, Park Y, Hwang GS, Ryu JS, Chang TS. Kaempferol suppresses collagen-induced platelet activation by inhibiting NADPH oxidase and protecting SHP-2 from oxidative inactivation. Free Radic Biol Med. 2015; 83:41–53.23. James TN, St Martin E, Willis PW 3rd, Lohr TO. Apoptosis as a possible cause of gradual development of complete heart block and fatal arrhythmias associated with absence of the AV node, sinus node, and internodal pathways. Circulation. 1996; 93:1424–1438.24. Hoogaars WM, Engel A, Brons JF, Verkerk AO, de Lange FJ, Wong LY, Bakker ML, Clout DE, Wakker V, Barnett P, Ravesloot JH, Moorman AF, Verheijck EE, Christoffels VM. Tbx3 controls the sinoatrial node gene program and imposes pacemaker function on the atria. Genes Dev. 2007; 21:1098–1112.25. Myers FB, Silver JS, Zhuge Y, Beygui RE, Zarins CK, Lee LP, Abilez OJ. Robust pluripotent stem cell expansion and cardiomyocyte differentiation via geometric patterning. Integr Biol (Camb). 2013; 5:1495–1506.26. Lian X, Hsiao C, Wilson G, Zhu K, Hazeltine LB, Azarin SM, Raval KK, Zhang J, Kamp TJ, Palecek SP. Robust cardiomyocyte differentiation from human pluripotent stem cells via temporal modulation of canonical Wnt signaling. Proc Natl Acad Sci U S A. 2012; 109:E1848–E1857.27. Jordan JL, Yamaguchi I, Mandel WJ. Studies on the mechanism of sinus node dysfunction in the sick sinus syndrome. Circulation. 1978; 57:217–223.28. Nitardy A, Langreck H, Dietz R, Stockburger M. Reduction of right ventricular pacing in patients with sinus node dysfunction through programming a long atrioventricular delay along with the DDIR mode. Clin Res Cardiol. 2009; 98:25–32.29. Le Scouarnec S, Bhasin N, Vieyres C, Hund TJ, Cunha SR, Koval O, Marionneau C, Chen B, Wu Y, Demolombe S, Song LS, Le Marec H, Probst V, Schott JJ, Anderson ME, Mohler PJ. Dysfunction in ankyrin-B-dependent ion channel and transporter targeting causes human sinus node disease. Proc Natl Acad Sci U S A. 2008; 105:15617–15622.30. Nof E, Antzelevitch C, Glikson M. The contribution of HCN4 to normal sinus node function in humans and animal models. Pacing Clin Electrophysiol. 2010; 33:100–106.31. Lee JM, Kalman JM. Sinus node dysfunction and atrial fibrillation: two sides of the same coin? Europace. 2013; 15:161–162.32. Kim DS, Ha KC, Kwon DY, Kim MS, Kim HR, Chae SW, Chae HJ. Kaempferol protects ischemia/reperfusion-induced cardiac damage through the regulation of endoplasmic reticulum stress. Immunopharmacol Immunotoxicol. 2008; 30:257–270.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Insulin sensitization causes accelerated sinus nodal dysfunction through autophagic dysregulation in hypertensive mice
- Sinus Node Dysfunction with Pulmonary Edema Associated with Hyponatremia
- Permanent Pacemaker for Syncope after Heart Transplantation with Bicaval Technique
- A case of sinus node dysfunction and atrial tachycardia after the excision of a left atrial myxoma
- Successful Treatment of Ischemic Dysfunction of the Sinus Node with Thrombolytic Therapy: A Case Report