Cancer Res Treat.
2015 Apr;47(2):274-281. 10.4143/crt.2014.025.
Prognostic Value of Splenic Artery Invasion in Patients Undergoing Adjuvant Chemoradiotherapy after Distal Pancreatectomy for Pancreatic Adenocarcinoma
- Affiliations
-
- 1Department of Radiation Oncology, Seoul National University College of Medicine, Seoul, Korea. kyubokim@snu.ac.kr
- 2Institute of Radiation Medicine, Medical Research Center, Seoul National University, Seoul, Korea.
- 3Department of Surgery, Seoul National University College of Medicine, Seoul, Korea.
- 4Department of Internal Medicine, Seoul National University College of Medicine, Seoul, Korea.
- 5Department of Radiology, Seoul National University College of Medicine, Seoul, Korea.
- KMID: 2132806
- DOI: http://doi.org/10.4143/crt.2014.025
Abstract
- PURPOSE
The purpose of this study was to evaluate the outcome of adjuvant chemoradiotherapy (CRT) after distal pancreatectomy (DP) in patients with pancreatic adenocarcinoma, and to identify the prognostic factors for these patients.
MATERIALS AND METHODS
We performed a retrospective review of 62 consecutive patients who underwent curative DP followed by adjuvant CRT between 2000 and 2011. There were 31 men and 31 women, and the median age was 64 years (range, 38 to 80 years). Adjuvant radiotherapy was delivered to the tumor bed and regional lymph nodes with a median dose of 50.4 Gy (range, 40 to 55.8 Gy). All patients received concomitant chemotherapy, and 53 patients (85.5%) also received maintenance chemotherapy. The median follow-up period was 24 months.
RESULTS
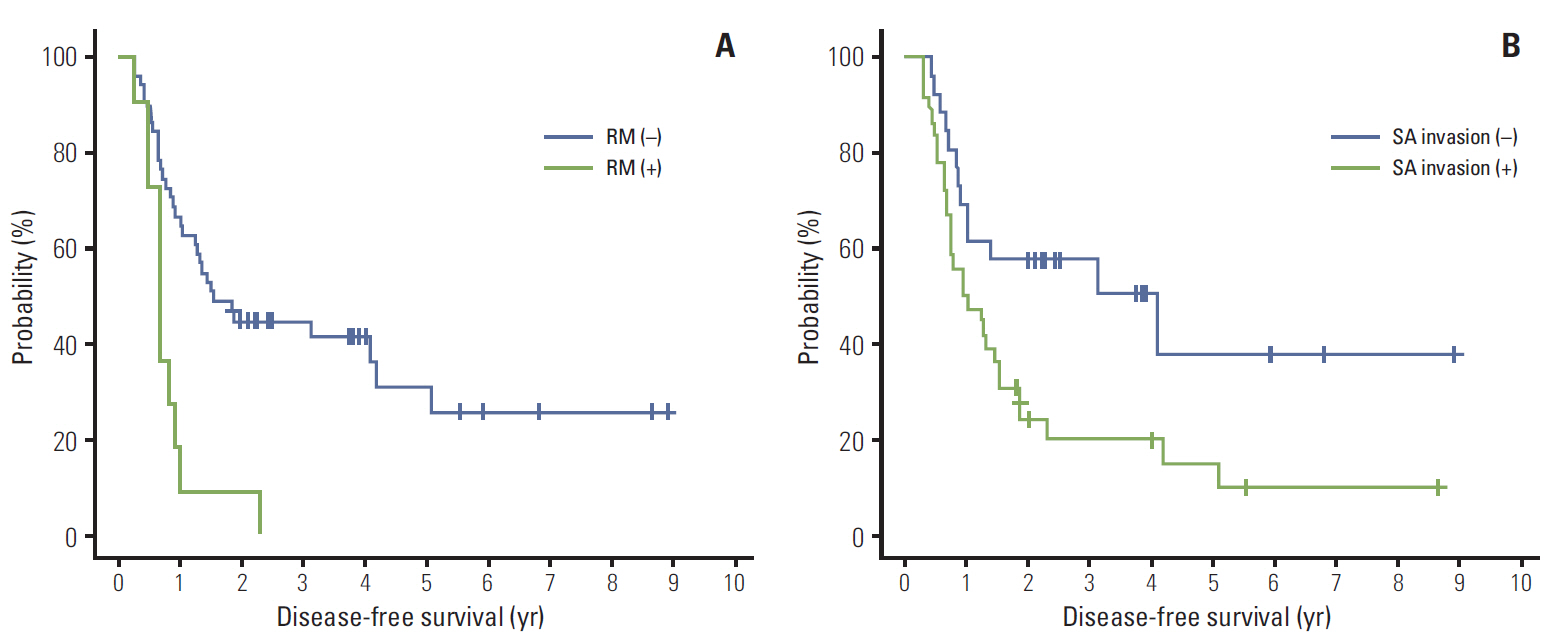
Forty patients (64.5%) experienced relapse. Isolated locoregional recurrence developed in 5 patients (8.1%) and distant metastasis in 35 patients (56.5%), of whom 13 had both locoregional recurrence and distant metastasis. The median overall survival (OS) and disease-free survival (DFS) were 37.5 months and 15.4 months, respectively. On multivariate analysis, splenic artery (SA) invasion (p=0.0186) and resection margin (RM) involvement (p=0.0004) were identified as significant adverse prognosticators for DFS. Also, male gender (p=0.0325) and RM involvement (p=0.0007) were associated with a significantly poor OS. Grade 3 or higher hematologic and gastrointestinal toxicities occurred in 22.6% and 4.8% of patients, respectively.
CONCLUSION
Adjuvant CRT may improve survival after DP for pancreatic body or tail adenocarcinoma. Our results indicated that SA invasion was a significant factor predicting inferior DFS, as was RM involvement. When SA invasion is identified preoperatively, neoadjuvant treatment may be considered.
MeSH Terms
-
Adenocarcinoma*
Chemoradiotherapy, Adjuvant*
Disease-Free Survival
Drug Therapy
Female
Follow-Up Studies
Humans
Lymph Nodes
Maintenance Chemotherapy
Male
Multivariate Analysis
Neoadjuvant Therapy
Neoplasm Metastasis
Pancreatectomy*
Pancreatic Neoplasms
Radiotherapy, Adjuvant
Recurrence
Retrospective Studies
Splenic Artery*
Figure
Reference
-
References
1. Garcea G, Dennison AR, Pattenden CJ, Neal CP, Sutton CD, Berry DP. Survival following curative resection for pancreatic ductal adenocarcinoma. A systematic review of the literature. JOP. 2008; 9:99–132.2. Van den Broeck A, Sergeant G, Ectors N, Van Steenbergen W, Aerts R, Topal B. Patterns of recurrence after curative resection of pancreatic ductal adenocarcinoma. Eur J Surg Oncol. 2009; 35:600–4.3. Kalser MH, Ellenberg SS. Pancreatic cancer. Adjuvant combined radiation and chemotherapy following curative resection. Arch Surg. 1985; 120:899–903.4. Klinkenbijl JH, Jeekel J, Sahmoud T, van Pel R, Couvreur ML, Veenhof CH, et al. Adjuvant radiotherapy and 5-fluorouracil after curative resection of cancer of the pancreas and periampullary region: phase III trial of the EORTC gastrointestinal tract cancer cooperative group. Ann Surg. 1999. 230:776–82.5. Neoptolemos JP, Dunn JA, Stocken DD, Almond J, Link K, Beger H, et al. Adjuvant chemoradiotherapy and chemotherapy in resectable pancreatic cancer: a randomised controlled trial. Lancet. 2001; 358:1576–85.6. Oettle H, Post S, Neuhaus P, Gellert K, Langrehr J, Ridwelski K, et al. Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: a randomized controlled trial. JAMA. 2007; 297:267–77.7. Neoptolemos JP, Stocken DD, Bassi C, Ghaneh P, Cunningham D, Goldstein D, et al. Adjuvant chemotherapy with fluorouracil plus folinic acid vs gemcitabine following pancreatic cancer resection: a randomized controlled trial. JAMA. 2010; 304:1073–81.8. Regine WF, Winter KA, Abrams R, Safran H, Hoffman JP, Konski A, et al. Fluorouracil-based chemoradiation with either gemcitabine or fluorouracil chemotherapy after resection of pancreatic adenocarcinoma: 5-year analysis of the U.S. Intergroup/RTOG 9704 phase III trial. Ann Surg Oncol. 2011; 18:1319–26.9. Van Laethem JL, Hammel P, Mornex F, Azria D, Van Tienhoven G, Vergauwe P, et al. Adjuvant gemcitabine alone versus gemcitabine-based chemoradiotherapy after curative resection for pancreatic cancer: a randomized EORTC-40013-22012/FFCD-9203/GERCOR phase II study. J Clin Oncol. 2010; 28:4450–6.10. Merchant NB, Rymer J, Koehler EA, Ayers GD, Castellanos J, Kooby DA, et al. Adjuvant chemoradiation therapy for pancreatic adenocarcinoma: who really benefits? J Am Coll Surg. 2009; 208:829–38.11. Corsini MM, Miller RC, Haddock MG, Donohue JH, Farnell MB, Nagorney DM, et al. Adjuvant radiotherapy and chemotherapy for pancreatic carcinoma: the Mayo Clinic experience (1975-2005). J Clin Oncol. 2008; 26:3511–6.12. Hsu CC, Herman JM, Corsini MM, Winter JM, Callister MD, Haddock MG, et al. Adjuvant chemoradiation for pancreatic adenocarcinoma: the Johns Hopkins Hospital-Mayo Clinic collaborative study. Ann Surg Oncol. 2010; 17:981–90.13. Christein JD, Kendrick ML, Iqbal CW, Nagorney DM, Farnell MB. Distal pancreatectomy for resectable adenocarcinoma of the body and tail of the pancreas. J Gastrointest Surg. 2005; 9:922–7.14. Sperti C, Pasquali C, Pedrazzoli S. Ductal adenocarcinoma of the body and tail of the pancreas. J Am Coll Surg. 1997; 185:255–9.15. Shoup M, Conlon KC, Klimstra D, Brennan MF. Is extended resection for adenocarcinoma of the body or tail of the pancreas justified? J Gastrointest Surg. 2003; 7:946–52.16. Brennan MF, Moccia RD, Klimstra D. Management of adenocarcinoma of the body and tail of the pancreas. Ann Surg. 1996; 223:506–11.17. Redmond KJ, Wolfgang CL, Sugar EA, Ahn J, Nathan H, Laheru D, et al. Adjuvant chemoradiation therapy for adenocarcinoma of the distal pancreas. Ann Surg Oncol. 2010; 17:3112–9.18. Hattangadi JA, Hong TS, Yeap BY, Mamon HJ. Results and patterns of failure in patients treated with adjuvant combined chemoradiation therapy for resected pancreatic adenocarcinoma. Cancer. 2009; 115:3640–50.19. Shimada K, Sakamoto Y, Sano T, Kosuge T. Prognostic factors after distal pancreatectomy with extended lymphadenectomy for invasive pancreatic adenocarcinoma of the body and tail. Surgery. 2006; 139:288–95.20. Kanda M, Fujii T, Sahin TT, Kanzaki A, Nagai S, Yamada S, et al. Invasion of the splenic artery is a crucial prognostic factor in carcinoma of the body and tail of the pancreas. Ann Surg. 2010; 251:483–7.21. Partelli S, Crippa S, Barugola G, Tamburrino D, Capelli P, D'Onofrio M, et al. Splenic artery invasion in pancreatic adenocarcinoma of the body and tail: a novel prognostic parameter for patient selection. Ann Surg Oncol. 2011; 18:3608–14.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Role of Distal Pancreatectomy on the Prognosis of Gastric Cancer Patients Undergoing Total Gastrectomy
- A Gastric Hemorrhage through the Fistula between Stomach and Pancreatic Pseudocyst with Ruptured Splenic Artery Pseudoaneurysm : A Case Report
- Adjuvant Chemotherapy Versus Chemoradiation for Patients with Resected Pancreatic Adenocarcinoma: A Single-Center Retrospective Study
- Laparoscopic Spleen Preserving Distal Pancreatectomy with the Conservation of the Splenic Artery and the Vein
- A Malignant Solid Pseudopapillary Tumor of the Pancreas in a 14-year-old Girl