Cancer Res Treat.
2015 Apr;47(2):221-241. 10.4143/crt.2013.159.
Radiation-Induced Autophagy Contributes to Cell Death and Induces Apoptosis Partly in Malignant Glioma Cells
- Affiliations
-
- 1Specific Organs Cancer Branch, National Cancer Center, Goyang, Korea.
- 2Department of Neurosurgery, University of Texas, MD Anderson Cancer Center, Houston, TX, USA.
- 3Department of Microbiology, Ajou University School of Medicine, Suwon, Korea.
- 4Cancer Cell and Molecular Biology Branch, National Cancer Center, Goyang, Korea.
- 5Neuro-oncology Clinic, National Cancer Center, Goyang, Korea. nsghs@ncc.re.kr
- KMID: 2132801
- DOI: http://doi.org/10.4143/crt.2013.159
Abstract
- PURPOSE
Radiation-induced autophagy has been shown to play two different roles, in malignant glioma (MG) cells, cytocidal or cytoprotective. However, neither the role of radiation-induced autophagy for cell death nor the existence of autophagy-induced apoptosis, a well-known cell-death pathway after irradiation, has been verified yet.
MATERIALS AND METHODS
We observed both temporal and dose-dependent response patterns of autophagy and apoptosis to radiation in MG cell lines. Additionally, we investigated the role of autophagy in apoptosis through knockdown of autophagy-related proteins.
RESULTS
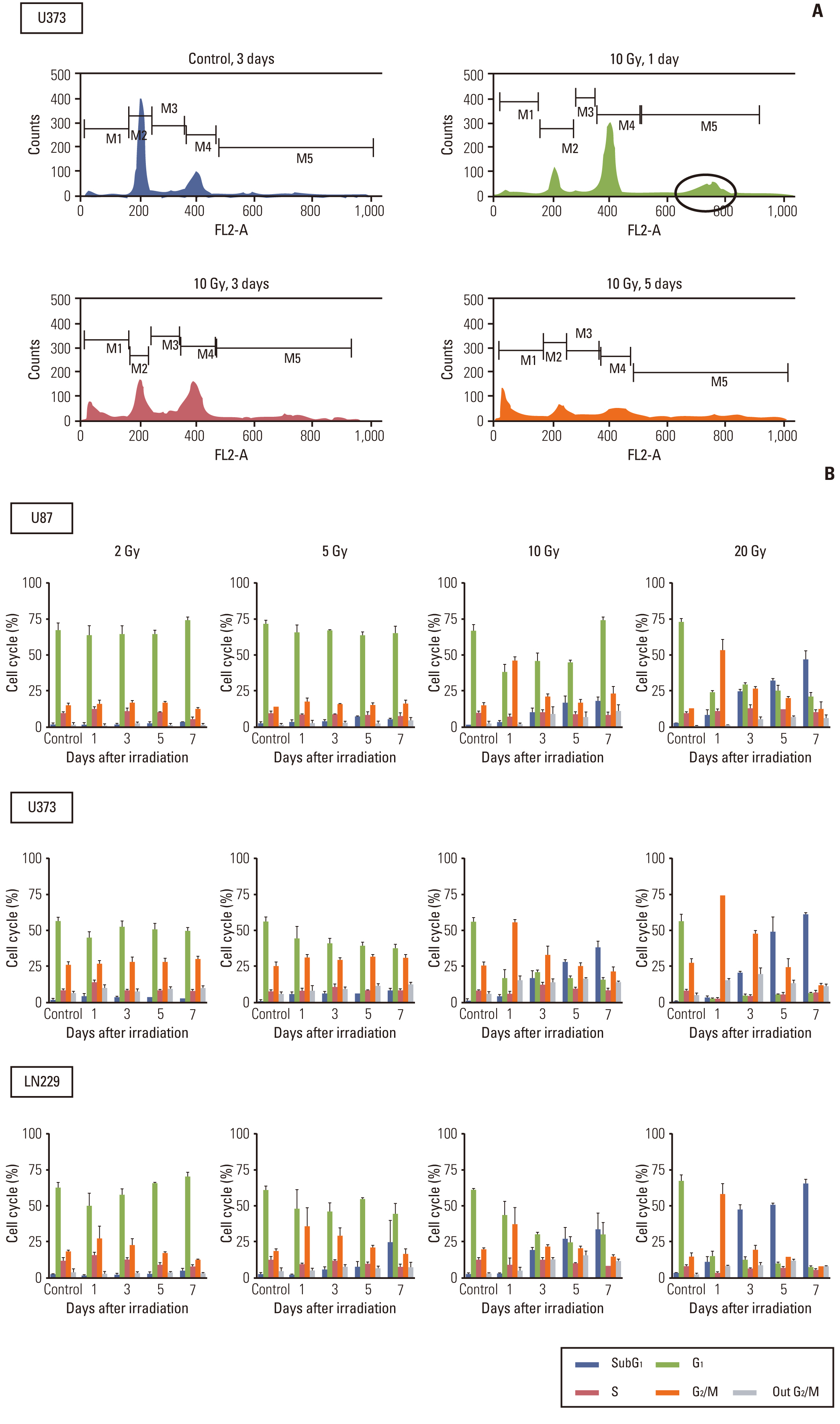
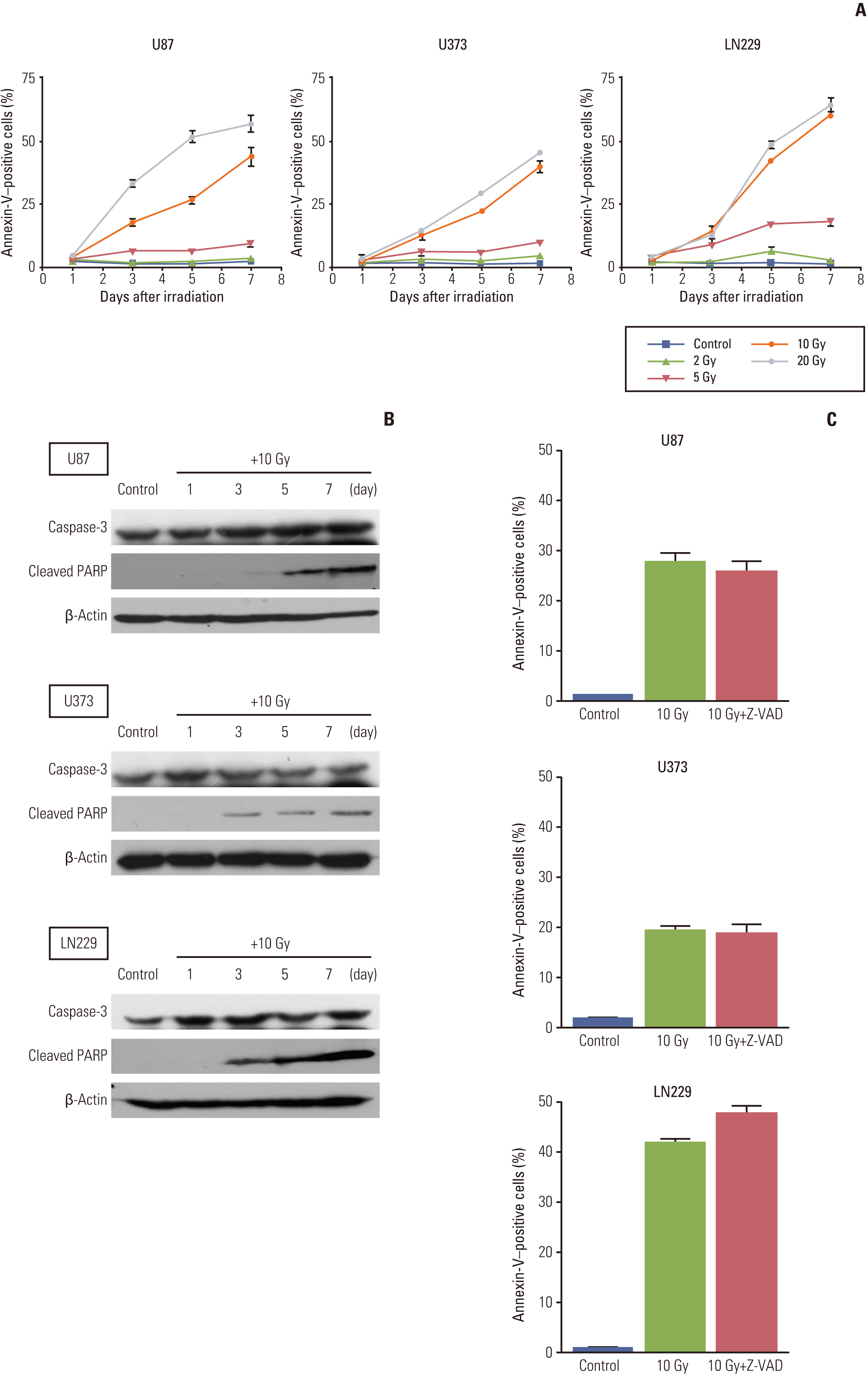
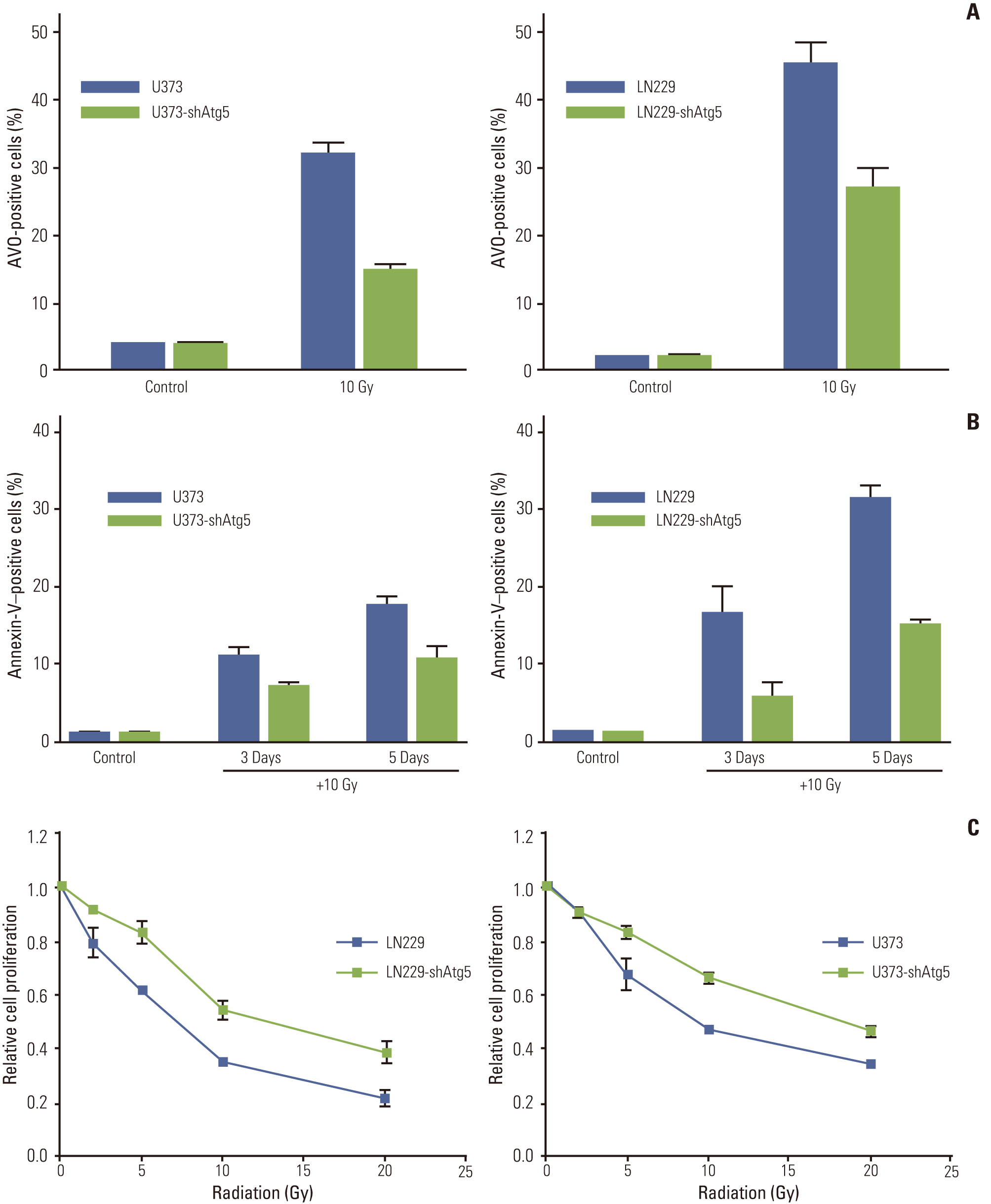
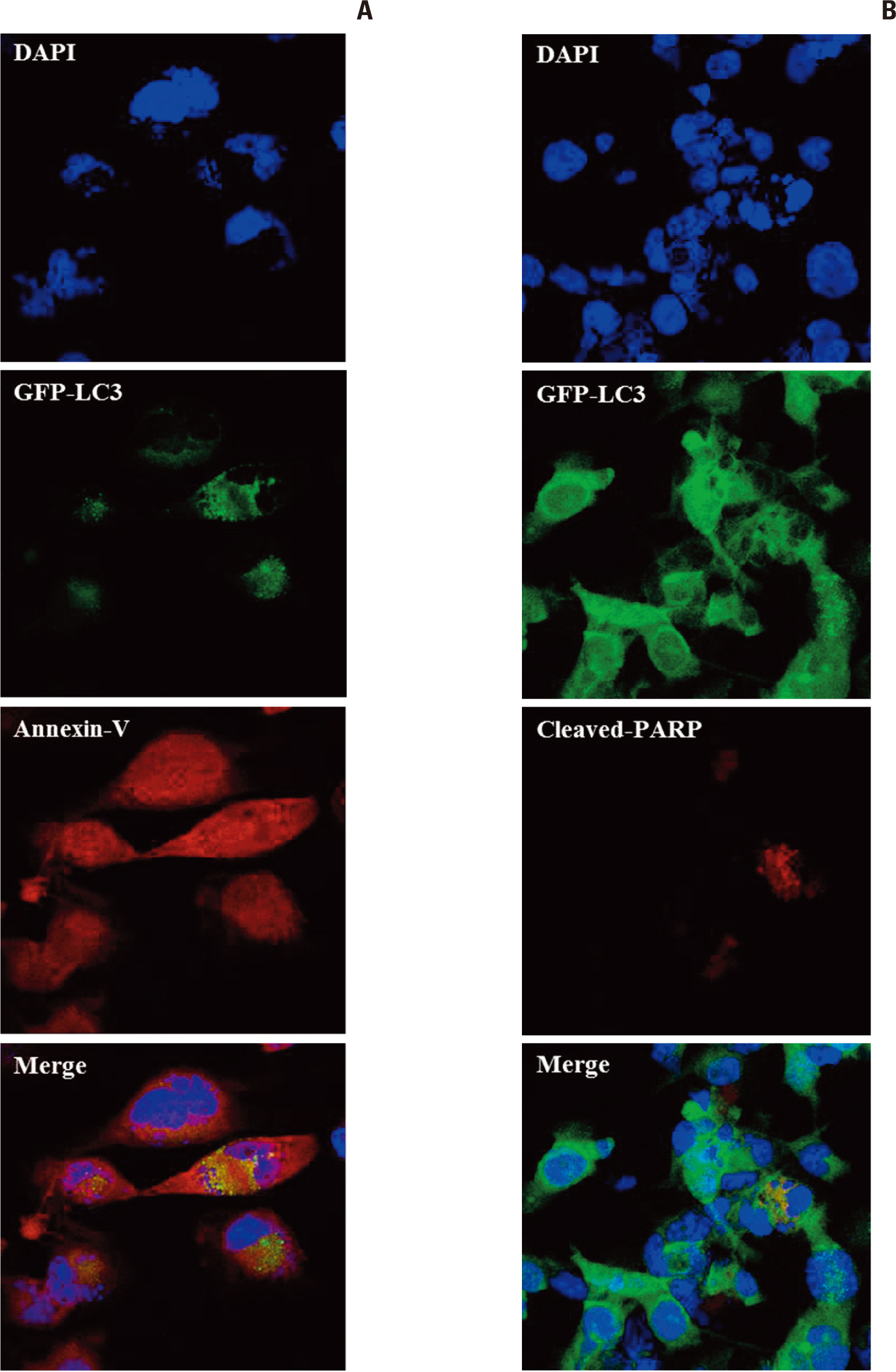
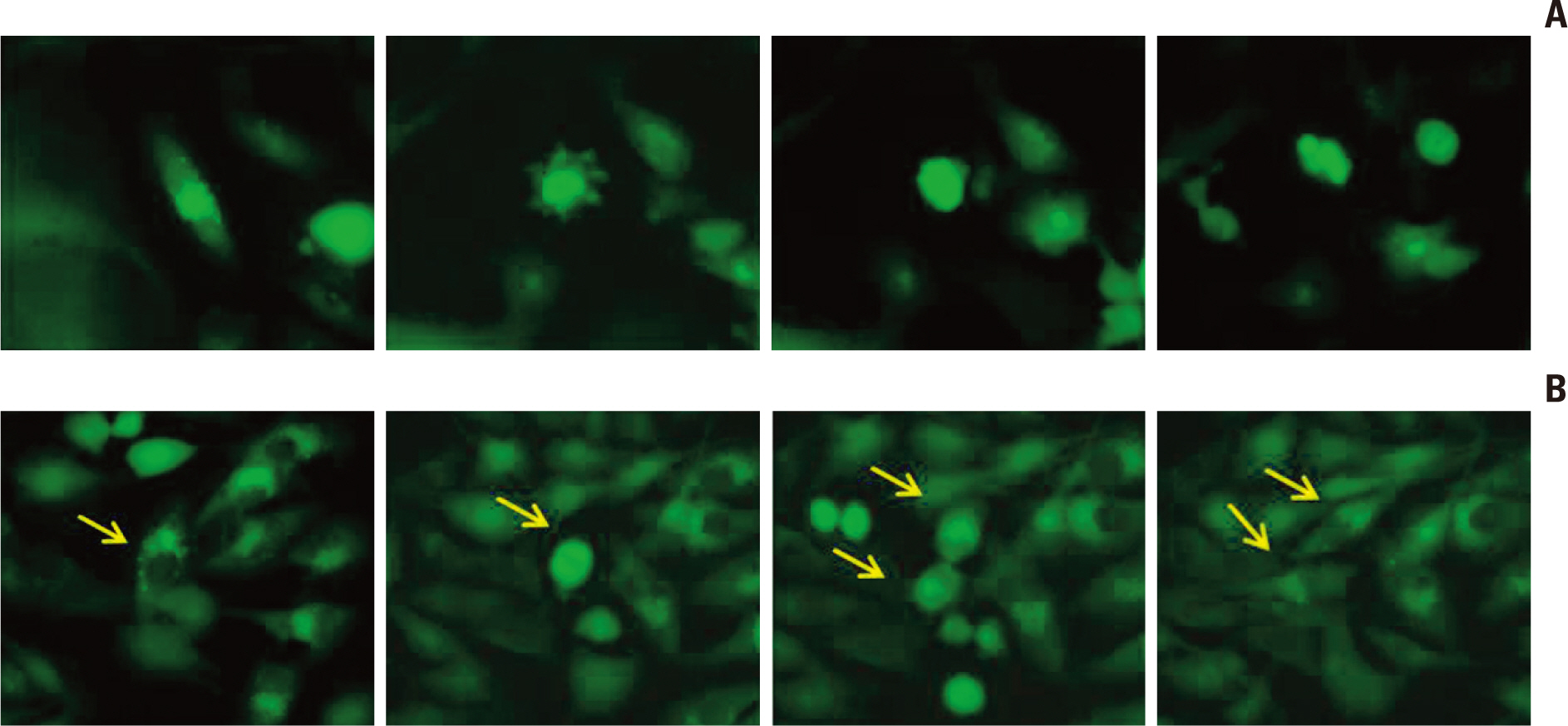
Autophagic activity measured by staining of acidic vesicle organelles and Western blotting of LC-3 protein increased in proportion to radiation dose from day 1 to 5 after irradiation. Apoptosis measured by annexin-V staining and Western blotting of cleaved poly(ADP-ribose) polymerase demonstrated relatively late appearance 3 days after irradiation that increased for up to 7 days. Blocking of pan-caspase (Z-VAD-FMK) did not affect apoptosis after irradiation, but silencing of Atg5 effectively reduced radiation-induced autophagy, which decreased apoptosis significantly. Inhibition of autophagy in Atg5 knockdown cells was shown to be beneficial for cell survival. Stable transfection of GFP-LC3 cells was observed after irradiation. Annexin-V was localized in cells bearing GFP-LC3 punctuated spots, indicating autophagy in immunofluorescence. Some of these punctuated GFP-LC3 bearing cells formed conglomerated spots and died in final phase.
CONCLUSION
These findings suggest that autophagy appears earlier than apoptosis after irradiation and that a portion of the apoptotic population that appears later is autophagy-dependent. Thus, autophagy is a pathway to cell death after irradiation of MG cells.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Dexamethasone Interferes with Autophagy and Affects Cell Survival in Irradiated Malignant Glioma Cells
Alfred Komakech, Ji-Hye Im, Ho-Shin Gwak, Kyue-Yim Lee, Jong Heon Kim, Byong Chul Yoo, Heesun Cheong, Jong Bae Park, Ji Woong Kwon, Sang Hoon Shin, Heon Yoo
J Korean Neurosurg Soc. 2020;63(5):566-578. doi: 10.3340/jkns.2019.0187.
Reference
-
References
1. Walker MD, Strike TA, Sheline GE. An analysis of dose-effect relationship in the radiotherapy of malignant gliomas. Int J Radiat Oncol Biol Phys. 1979; 5:1725–31.
Article2. Hochberg FH, Pruitt A. Assumptions in the radiotherapy of glioblastoma. Neurology. 1980; 30:907–11.
Article3. Olive PL, Durand RE. Apoptosis: an indicator of radiosensitivity in vitro? Int J Radiat Biol. 1997; 71:695–707.4. Shinomiya N. New concepts in radiation-induced apoptosis: 'premitotic apoptosis' and 'postmitotic apoptosis'. J Cell Mol Med. 2001; 5:240–53.
Article5. Ning S, Knox SJ. G2/M-phase arrest and death by apoptosis of HL60 cells irradiated with exponentially decreasing low-doserate gamma radiation. Radiat Res. 1999; 151:659–69.6. Kondo Y, Kanzawa T, Sawaya R, Kondo S. The role of autophagy in cancer development and response to therapy. Nat Rev Cancer. 2005; 5:726–34.
Article7. Shintani T, Klionsky DJ. Autophagy in health and disease: a double-edged sword. Science. 2004; 306:990–5.
Article8. Lambert LA, Qiao N, Hunt KK, Lambert DH, Mills GB, Meijer L, et al. Autophagy: a novel mechanism of synergistic cytotoxicity between doxorubicin and roscovitine in a sarcoma model. Cancer Res. 2008; 68:7966–74.
Article9. Paglin S, Hollister T, Delohery T, Hackett N, McMahill M, Sphicas E, et al. A novel response of cancer cells to radiation involves autophagy and formation of acidic vesicles. Cancer Res. 2001; 61:439–44.10. Yao KC, Komata T, Kondo Y, Kanzawa T, Kondo S, Germano IM. Molecular response of human glioblastoma multiforme cells to ionizing radiation: cell cycle arrest, modulation of the expression of cyclin-dependent kinase inhibitors, and autophagy. J Neurosurg. 2003; 98:378–84.11. Ito H, Daido S, Kanzawa T, Kondo S, Kondo Y. Radiation-induced autophagy is associated with LC3 and its inhibition sensitizes malignant glioma cells. Int J Oncol. 2005; 26:1401–10.
Article12. Yousefi S, Perozzo R, Schmid I, Ziemiecki A, Schaffner T, Scapozza L, et al. Calpain-mediated cleavage of Atg5 switches autophagy to apoptosis. Nat Cell Biol. 2006; 8:1124–32.
Article13. Luo S, Rubinsztein DC. Atg5 and Bcl-2 provide novel insights into the interplay between apoptosis and autophagy. Cell Death Differ. 2007; 14:1247–50.
Article14. Kanzawa T, Germano IM, Komata T, Ito H, Kondo Y, Kondo S. Role of autophagy in temozolomide-induced cytotoxicity for malignant glioma cells. Cell Death Differ. 2004; 11:448–57.
Article15. Fowler JF. The linear-quadratic formula and progress in fractionated radiotherapy. Br J Radiol. 1989; 62:679–94.
Article16. Shinomiya N, Kuno Y, Yamamoto F, Fukasawa M, Okumura A, Uefuji M, et al. Different mechanisms between premitotic apoptosis and postmitotic apoptosis in X-irradiated U937 cells. Int J Radiat Oncol Biol Phys. 2000; 47:767–77.
Article17. Su LN, Little JB. Prolonged cell cycle delay in radioresistant human cell lines transfected with activated ras oncogene and/or simian virus 40 T-antigen. Radiat Res. 1993; 133:73–9.
Article18. Stuschke M, Sak A, Wurm R, Sinn B, Wolf G, Stuben G, et al. Radiation-induced apoptosis in human non-small-cell lung cancer cell lines is secondary to cell-cycle progression beyond the G2-phase checkpoint. Int J Radiat Biol. 2002; 78:807–19.
Article19. Broker LE, Huisman C, Ferreira CG, Rodriguez JA, Kruyt FA, Giaccone G. Late activation of apoptotic pathways plays a negligible role in mediating the cytotoxic effects of discodermolide and epothilone B in non-small cell lung cancer cells. Cancer Res. 2002; 62:4081–8.20. Cummings BS, Kinsey GR, Bolchoz LJ, Schnellmann RG. Identification of caspase-independent apoptosis in epithelial and cancer cells. J Pharmacol Exp Ther. 2004; 310:126–34.
Article21. Chaitanya GV, Babu PP. Differential PARP cleavage: an indication of heterogeneous forms of cell death and involvement of multiple proteases in the infarct of focal cerebral ischemia in rat. Cell Mol Neurobiol. 2009; 29:563–73.
Article22. Gwak HS, Shingu T, Chumbalkar V, Hwang YH, DeJournett R, Latha K, et al. Combined action of the dinuclear platinum compound BBR3610 with the PI3-K inhibitor PX-866 in glioblastoma. Int J Cancer. 2011; 128:787–96.
Article23. Codogno P, Meijer AJ. Atg5: more than an autophagy factor. Nat Cell Biol. 2006; 8:1045–7.
Article24. Shimizu S, Kanaseki T, Mizushima N, Mizuta T, Arakawa-Kobayashi S, Thompson CB, et al. Role of Bcl-2 family proteins in a non-apoptotic programmed cell death dependent on autophagy genes. Nat Cell Biol. 2004; 6:1221–8.
Article25. Kim KW, Mutter RW, Cao C, Albert JM, Freeman M, Hallahan DE, et al. Autophagy for cancer therapy through inhibition of pro-apoptotic proteins and mammalian target of rapamycin signaling. J Biol Chem. 2006; 281:36883–90.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Dexamethasone Interferes with Autophagy and Affects Cell Survival in Irradiated Malignant Glioma Cells
- Memantine Induces NMDAR1-Mediated Autophagic Cell Death in Malignant Glioma Cells
- Regulatory Role of Autophagy in Globular Adiponectin-Induced Apoptosis in Cancer Cells
- Induction of Apoptosis and Autophagy in UVB-Treated HaCaT Cells
- The Impact of Autophagy on the Cigarette Smoke Extract-Induced Apoptosis of Bronchial Epithelial Cells