Cancer Res Treat.
2015 Apr;47(2):173-181. 10.4143/crt.2014.055.
Treatment Outcomes of Rituximab Plus Hyper-CVAD in Korean Patients with Sporadic Burkitt or Burkitt-like Lymphoma: Results of a Multicenter Analysis
- Affiliations
-
- 1Department of Internal Medicine, Gachon University Gil Medical Center, Incheon, Korea.
- 2Department of Medicine, Samsung Medical Center, Sunkyunkwan University School of Medicine, Seoul, Korea. kstwoh@skku.edu
- 3Department of Hematology-Oncology, Chonnam National University Hwasun Hospital, Hwasun, Korea.
- 4Department of Hematology-Oncology, Pusan National University Hospital, Busan, Korea.
- 5Department of Internal Medicine, Gangnam Severance Hospital, Yonsei University College of Medicine, Seoul, Korea.
- 6Department of Internal Medicine, Kosin University Gospel Hospital, Busan, Korea.
- 7Department of Internal Medicine, Chonbuk National University Hospital, Jeonju, Korea.
- 8Department of Oncology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- 9Department of Internal Medicine, Yeungnam University Medical Center, Daegu, Korea.
- 10Department of Internal Medicine, Dong-A University Hospital, Busan, Korea.
- 11Department of Internal Medicine, Korea University College of Medicine, Seoul, Korea.
- 12Department of Internal Medicine, Ewha Womans University Mokdong Hospital, Seoul, Korea.
- 13Department of Hematology-Oncology, Keimyung University Dongsan Medical Center, Keimyung University School of Medicine, Daegu, Korea.
- 14Department of Internal Medicine, Daegu Catholic University Medical Center, Daegu, Korea.
- KMID: 2132795
- DOI: http://doi.org/10.4143/crt.2014.055
Abstract
- PURPOSE
This study was conducted to evaluate outcomes in adult patients with Burkitt lymphoma (BL) or Burkitt-like lymphoma treated with an rituximab plus hyper-CVAD (R-hyper-CVAD) regimen by focusing on tolerability and actual delivered relative dose intensity (RDI).
MATERIALS AND METHODS
Patients > or = 20 years of age and pathologically diagnosed with BL or Burkitt-like lymphoma were treated with at least one cycle of R-hyper-CVAD as the first-line treatment in this study. Eligible patients' case report forms were requested from their physicians to obtain clinical and laboratory data for this retrospective study.
RESULTS
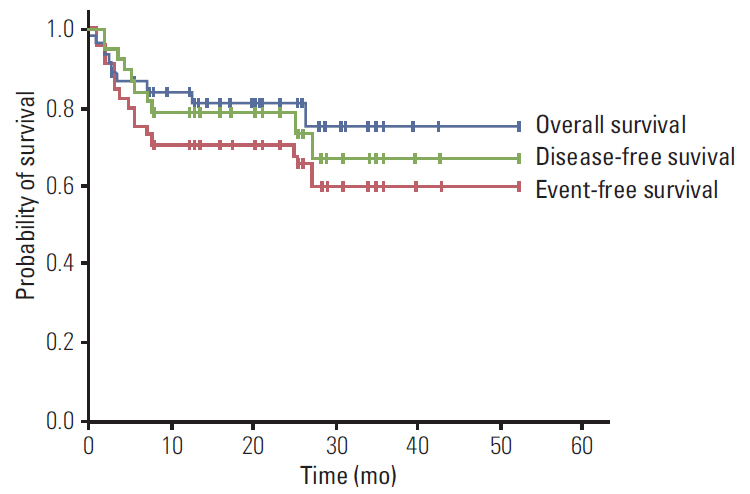
Forty-three patients (median age, 51 years) from 14 medical centers in Korea were analyzed, none of which were infected with human immunodeficiency virus. The majority of patients had advanced diseases, and 24 patients achieved a complete response (75.0%). After a median follow-up period of 20.0 months, 2-year event-free and overall survival rates were 70.9% and 81.4%, respectively. Eleven patients (25.6%) were unable to complete the R-hyper-CVAD regimen, including six patients due to early death. The RDIs of adriamycin, vincristine, methotrexate, and cytarabine were between 60% and 65%, which means less than 25% of patients received greater than 80% of the planned dose of each drug. Poor performance status was related to the lower RDIs of doxorubicin and methotrexate.
CONCLUSION
R-hyper-CVAD showed excellent treatment outcomes in patients who were suitable for dose-intense chemotherapy. However, management of patients who are intolerant to a dose-intense regimen remains problematic due to the frequent occurrence of treatmentrelated complications.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
The Consortium for Improving Survival of Lymphoma (CISL): recent achievements and future perspective
Cheolwon Suh, Byeong-Bae Park, Won Seog Kim
Blood Res. 2017;52(1):3-6. doi: 10.5045/br.2017.52.1.3.
Reference
-
References
1. Linch DC. Burkitt lymphoma in adults. Br J Haematol. 2012; 156:693–703.
Article2. Huh J. Epidemiologic overview of malignant lymphoma. Korean J Hematol. 2012; 47:92–104.
Article3. Magrath I. The pathogenesis of Burkitt's lymphoma. Adv Cancer Res. 1990; 55:133–270.
Article4. Ostronoff M, Soussain C, Zambon E, Ibrahim A, Bosq J, Bayle C, et al. Burkitt's lymphoma in adults: a retrospective study of 46 cases. Nouv Rev Fr Hematol. 1992; 34:389–97.5. Mead GM, Sydes MR, Walewski J, Grigg A, Hatton CS, Pescosta N, et al. An international evaluation of CODOX-M and CODOX-M alternating with IVAC in adult Burkitt's lymphoma: results of United Kingdom Lymphoma Group LY06 study. Ann Oncol. 2002; 13:1264–74.
Article6. Thomas DA, Cortes J, O'Brien S, Pierce S, Faderl S, Albitar M, et al. Hyper-CVAD program in Burkitt's-type adult acute lymphoblastic leukemia. J Clin Oncol. 1999; 17:2461–70.
Article7. Costa LJ, Xavier AC, Wahlquist AE, Hill EG. Trends in survival of patients with Burkitt lymphoma/leukemia in the USA: an analysis of 3691 cases. Blood. 2013; 121:4861–6.
Article8. Wasterlid T, Brown PN, Hagberg O, Hagberg H, Pedersen LM, D'Amore F, et al. Impact of chemotherapy regimen and rituximab in adult Burkitt lymphoma: a retrospective population-based study from the Nordic Lymphoma Group. Ann Oncol. 2013; 24:1879–86.9. Thomas DA, O'Brien S, Faderl S, Manning JT Jr, Romaguera J, Fayad L, et al. Burkitt lymphoma and atypical Burkitt or Burkitt-like lymphoma: should these be treated as different diseases? Curr Hematol Malig Rep. 2011; 6:58–66.
Article10. Corazzelli G, Frigeri F, Russo F, Frairia C, Arcamone M, Esposito G, et al. RD-CODOX-M/IVAC with rituximab and intrathecal liposomal cytarabine in adult Burkitt lymphoma and 'unclassifiable' highly aggressive B-cell lymphoma. Br J Haematol. 2012; 156:234–44.
Article11. Thomas DA, Faderl S, O'Brien S, Bueso-Ramos C, Cortes J, Garcia-Manero G, et al. Chemoimmunotherapy with hyper-CVAD plus rituximab for the treatment of adult Burkitt and Burkitt-type lymphoma or acute lymphoblastic leukemia. Cancer. 2006; 106:1569–80.
Article12. Suh C, Kim WS, Kim JS, Park BB. Review of the clinical research conducted by the Consortium for Improving Survival of Lymphoma of the Korean Society of Hematology Lymphoma Working Party. Blood Res. 2013; 48:171–7.
Article13. Cheson BD, Pfistner B, Juweid ME, Gascoyne RD, Specht L, Horning SJ, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007; 25:579–86.
Article14. Soussain C, Patte C, Ostronoff M, Delmer A, Rigal-Huguet F, Cambier N, et al. Small noncleaved cell lymphoma and leukemia in adults. A retrospective study of 65 adults treated with the LMB pediatric protocols. Blood. 1995; 85:664–74.
Article15. Lee EJ, Petroni GR, Schiffer CA, Freter CE, Johnson JL, Barcos M, et al. Brief-duration high-intensity chemotherapy for patients with small noncleaved-cell lymphoma or FAB L3 acute lymphocytic leukemia: results of cancer and leukemia group B study 9251. J Clin Oncol. 2001; 19:4014–22.
Article16. Mead GM, Barrans SL, Qian W, Walewski J, Radford JA, Wolf M, et al. A prospective clinicopathologic study of dose-modified CODOX-M/IVAC in patients with sporadic Burkitt lymphoma defined using cytogenetic and immunophenotypic criteria (MRC/NCRI LY10 trial). Blood. 2008; 112:2248–60.
Article17. Choi MK, Jun HJ, Lee SY, Kim KH, Lim DH, Kim K, et al. Treatment outcome of adult patients with Burkitt lymphoma: results using the LMB protocol in Korea. Ann Hematol. 2009; 88:1099–106.
Article18. Barnes JA, Lacasce AS, Feng Y, Toomey CE, Neuberg D, Michaelson JS, et al. Evaluation of the addition of rituximab to CODOX-M/IVAC for Burkitt's lymphoma: a retrospective analysis. Ann Oncol. 2011; 22:1859–64.
Article19. Intermesoli T, Rambaldi A, Rossi G, Delaini F, Romani C, Pogliani EM, et al. High cure rates in Burkitt lymphoma and leukemia: a Northern Italy Leukemia Group study of the German short intensive rituximab-chemotherapy program. Haematologica. 2013; 98:1718–25.
Article20. Maruyama D, Watanabe T, Maeshima AM, Nomoto J, Taniguchi H, Azuma T, et al. Modified cyclophosphamide, vincristine, doxorubicin, and methotrexate (CODOX-M)/ifosfamide, etoposide, and cytarabine (IVAC) therapy with or without rituximab in Japanese adult patients with Burkitt lymphoma (BL) and B cell lymphoma, unclassifiable, with features intermediate between diffuse large B cell lymphoma and BL. Int J Hematol. 2010; 92:732–43.
Article21. Freifeld AG, Bow EJ, Sepkowitz KA, Boeckh MJ, Ito JI, Mullen CA, et al. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the infectious diseases society of america. Clin Infect Dis. 2011; 52:e56–93.
Article22. Goldman SC, Holcenberg JS, Finklestein JZ, Hutchinson R, Kreissman S, Johnson FL, et al. A randomized comparison between rasburicase and allopurinol in children with lymphoma or leukemia at high risk for tumor lysis. Blood. 2001; 97:2998–3003.
Article23. Alvarnas JC, Brown PA, Aoun P, Ballen KK, Bellam N, Blum W, et al. Acute lymphoblastic leukemia. J Natl Compr Canc Netw. 2012; 10:858–914.
Article24. Kelly JL, Toothaker SR, Ciminello L, Hoelzer D, Holte H, La-Casce AS, et al. Outcomes of patients with Burkitt lymphoma older than age 40 treated with intensive chemotherapeutic regimens. Clin Lymphoma Myeloma. 2009; 9:307–10.
Article25. Dunleavy K, Pittaluga S, Shovlin M, Steinberg SM, Cole D, Grant C, et al. Low-intensity therapy in adults with Burkitt's lymphoma. N Engl J Med. 2013; 369:1915–25.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Sporadic Nasopharyngeal Burkitt's Lymphoma
- Primary Burkitt's Lymphoma in the Nasal Cavity and Paranasal Sinuses
- A Case of Mantle Cell Lymphoma Treated with Autologous Stem Cell Transplantation and Rituximab
- Burkitt Lymphoma
- Rehabilitation for Ataxia Following Chemotherapy for Burkitt Lymphoma Involving the Rectum