Allergy Asthma Immunol Res.
2011 Apr;3(2):96-102. 10.4168/aair.2011.3.2.96.
Pseudoceramide-Containing Physiological Lipid Mixture Reduces Adverse Effects of Topical Steroids
- Affiliations
-
- 1Department of Dermatology & Atopy Clinic, Seoul Medical Center, Seoul, Korea.
- 2Research Division, NeoPharm Co., Ltd., Daejeon, Korea.
- 3Department of Dermatology, Yonsei University Wonju College of Medicine, Wonju, Korea.
- 4Department of Dermatology, Yonsei University College of Medicine, Seoul, Korea. ydshderm@yuhs.ac
- KMID: 2130360
- DOI: http://doi.org/10.4168/aair.2011.3.2.96
Abstract
- PURPOSE
Various therapeutic approaches have been suggested for preventing or reducing the adverse effects of topical glucocorticoids, including skin barrier impairment. Previously, we have shown that impairment of skin barrier function by the highest potency topical glucocorticoid, clobetasol 17-propinate (CP), can be partially prevented by co-application of a physiological lipid mixture containing pseudoceramide, free fatty acids, and cholesterol (multi-lamellar emulsion [MLE]). Skin atrophic effects of CP were also partially reduced by MLE. In this study, the preventive effects of MLE on the lowest potency topical glucocorticoid, hydrocortisone (HC), were investigated using animal models.
METHODS
Anti-inflammatory activity of topical HC was evaluated using a 12-O-tetradecanoylphobol-13-acetate-induced skin edema model. Topical steroid induced adverse effects were evaluated using hairless mouse.
RESULTS
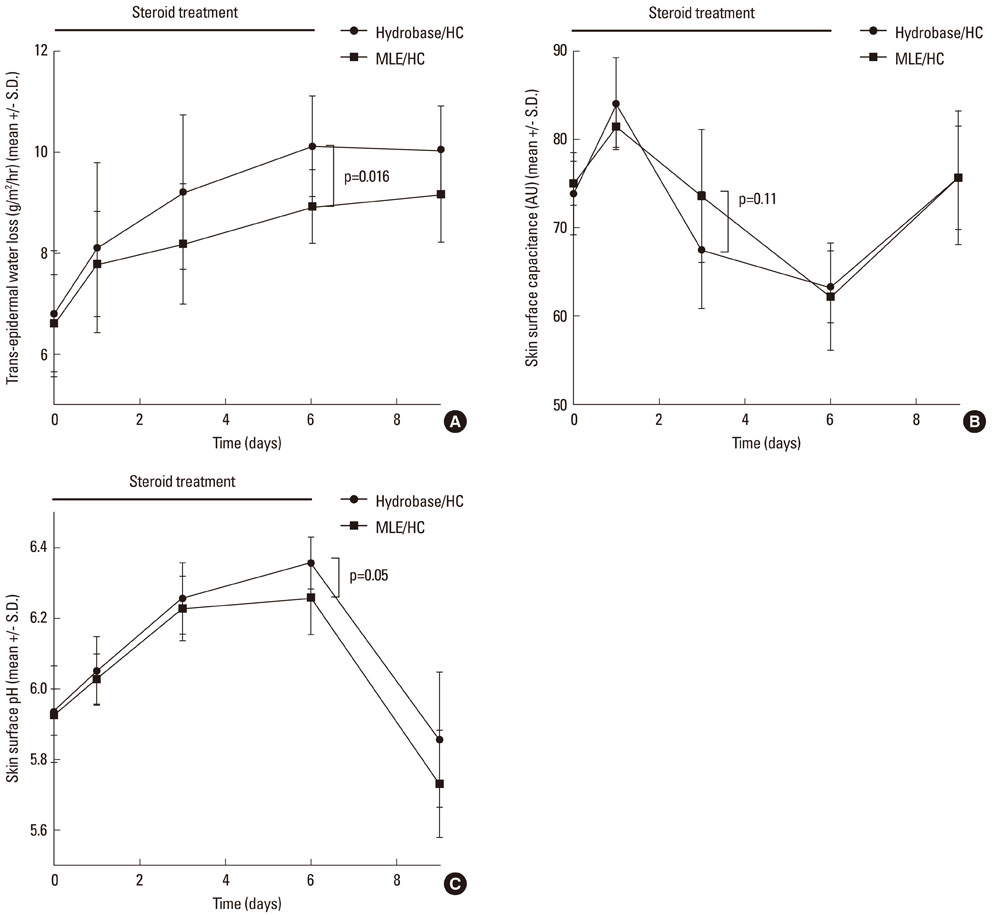
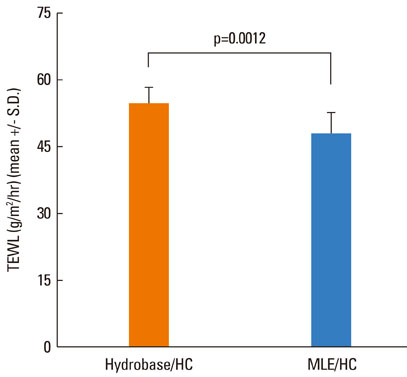
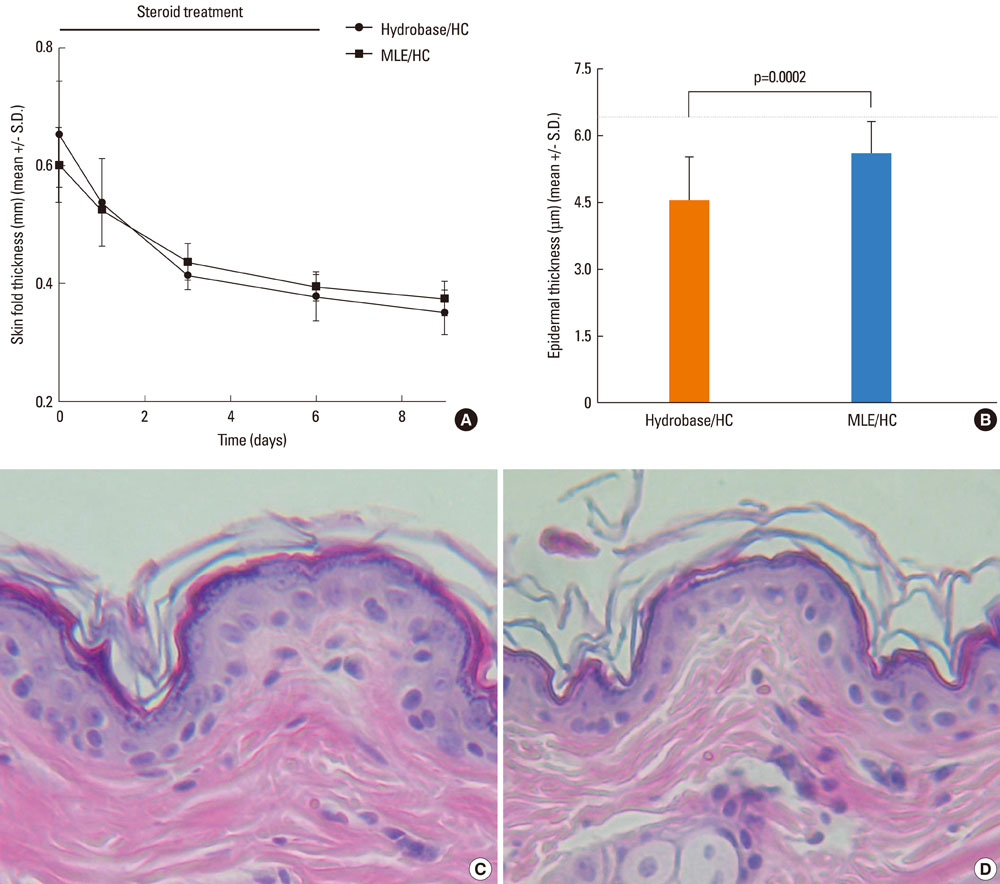
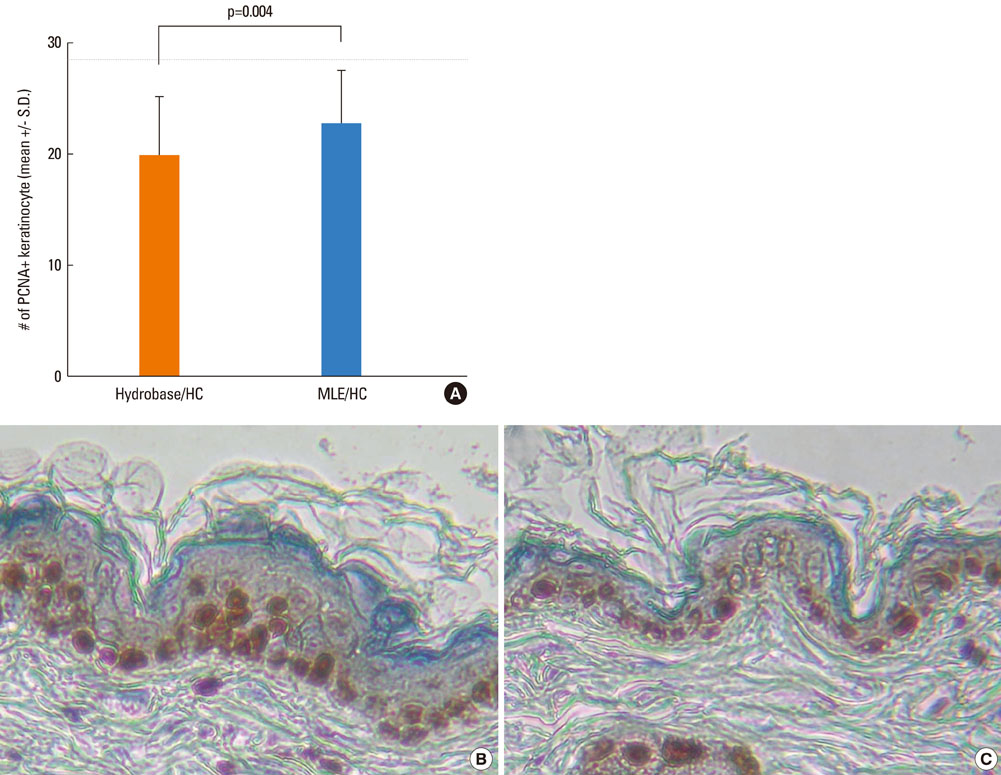
The results showed that the anti-inflammatory activity was not altered by co-application of either MLE or hydrobase. However, co-application of MLE and 1.0% HC showed less impairment in the epidermal permeability barrier function, skin hydration, and skin surface pH compared with hydrobase. Stratum corneum integrity, evaluated by measuring trans-epidermal water loss after repeated tape stripping, showed less damage with MLE co-application. Long-term application of topical HC induced skin atrophy, measured by a reduction in skinfold and epidermal thickness and in the number of epidermal proliferating cell nucleus antigen (PCNA)-positive keratinocytes. Co-application of MLE did not affect the skinfold or epidermal thickness, but the number of PCNA-positive keratinocytes was less decreased with MLE use.
CONCLUSIONS
These results suggest that co-application of MLE is effective in reducing the local adverse effects of low-potency topical glucocorticoids and supports the therapeutic efficacy of physiological lipid mixtures on skin barrier function.
MeSH Terms
Figure
Cited by 1 articles
-
The Clinical Efficacy of Mometasone Furoate in Multi-Lamellar Emulsion for Eczema: A Double-blinded Crossover Study
Duk Han Kim, Hyun Jong Lee, Chun Wook Park, Kyu Han Kim, Kwang Hoon Lee, Byung In Ro, Sang Hyun Cho
Ann Dermatol. 2013;25(1):17-22. doi: 10.5021/ad.2013.25.1.17.
Reference
-
1. Hengge UR, Ruzicka T, Schwartz RA, Cork MJ. Adverse effects of topical glucocorticosteroids. J Am Acad Dermatol. 2006. 54:1–15.2. Charman CR, Morris AD, Williams HC. Topical corticosteroid phobia in patients with atopic eczema. Br J Dermatol. 2000. 142:931–936.3. Fukaya M. Why do patients with atopic dermatitis refuse to apply topical corticosteroids? Dermatology. 2000. 201:242–245.4. Sheu HM, Lee JY, Chai CY, Kuo KW. Depletion of stratum corneum intercellular lipid lamellae and barrier function abnormalities after long-term topical corticosteroids. Br J Dermatol. 1997. 136:884–890.5. Kao JS, Fluhr JW, Man MQ, Fowler AJ, Hachem JP, Crumrine D, Ahn SK, Brown BE, Elias PM, Feingold KR. Short-term glucocorticoid treatment compromises both permeability barrier homeostasis and stratum corneum integrity: inhibition of epidermal lipid synthesis accounts for functional abnormalities. J Invest Dermatol. 2003. 120:456–464.6. Elias PM, Hatano Y, Williams ML. Basis for the barrier abnormality in atopic dermatitis: outside-inside-outside pathogenic mechanisms. J Allergy Clin Immunol. 2008. 121:1337–1343.7. Proksch E. The role of emollients in the management of diseases with chronic dry skin. Skin Pharmacol Physiol. 2008. 21:75–80.8. Lee SH, Jeong SK, Ahn SK. An update of the defensive barrier function of skin. Yonsei Med J. 2006. 47:293–306.9. Elias PM. Skin barrier function. Curr Allergy Asthma Rep. 2008. 8:299–305.10. Mao-Qiang M, Feingold KR, Thornfeldt CR, Elias PM. Optimization of physiological lipid mixtures for barrier repair. J Invest Dermatol. 1996. 106:1096–1101.11. Park BD, Youm JK, Jeong SK, Choi EH, Ahn SK, Lee SH. The characterization of molecular organization of multilamellar emulsions containing pseudoceramide and type III synthetic ceramide. J Invest Dermatol. 2003. 121:794–801.12. Rim JH, Jo SJ, Park JY, Park BD, Youn JI. Electrical measurement of moisturizing effect on skin hydration and barrier function in psoriasis patients. Clin Exp Dermatol. 2005. 30:409–413.13. Draelos ZD. The effect of ceramide-containing skin care products on eczema resolution duration. Cutis. 2008. 81:87–91.14. Ahn SK, Bak HN, Park BD, Kim YH, Youm JK, Choi EH, Hong SP, Lee SH. Effects of a multilamellar emulsion on glucocorticoid-induced epidermal atrophy and barrier impairment. J Dermatol. 2006. 33:80–90.15. Beattie PE, Lewis-Jones MS. Parental knowledge of topical therapies in the treatment of childhood atopic dermatitis. Clin Exp Dermatol. 2003. 28:549–553.16. Korting HC, Unholzer A, Schafer-Korting M, Tausch I, Gassmueller J, Nietsch KH. Different skin thinning potential of equipotent medium-strength glucocorticoids. Skin Pharmacol Appl Skin Physiol. 2002. 15:85–91.17. Tharp MD. A comparison of twice-daily and once-daily administration of fluticasone propionate cream, 0.05%, in the treatment of eczema. Cutis. 1996. 57:19–26.18. Kaidbey K, Kopper SC, Sefton J, Gibson JR. A pilot study to determine the effect of tazarotene gel 0.1% on steroid-induced epidermal atrophy. Int J Dermatol. 2001. 40:468–471.19. Sheu HM, Lee JY, Kuo KW, Tsai JC. Permeability barrier abnormality of hairless mouse epidermis after topical corticosteroid: characterization of stratum corneum lipids by ruthenium tetroxide staining and high-performance thin-layer chromatography. J Dermatol. 1998. 25:281–289.20. Elias PM. Stratum corneum defensive functions: an integrated view. J Invest Dermatol. 2005. 125:183–200.21. Abramovits W. Atopic dermatitis. J Am Acad Dermatol. 2005. 53:S86–S93.22. Elias PM, Schmuth M. Abnormal skin barrier in the etiopathogenesis of atopic dermatitis. Curr Opin Allergy Clin Immunol. 2009. 9:437–446.23. Demerjian M, Choi EH, Man MQ, Chang S, Elias PM, Feingold KR. Activators of PPARs and LXR decrease the adverse effects of exogenous glucocorticoids on the epidermis. Exp Dermatol. 2009. 18:643–649.24. Schmuth M, Jiang YJ, Dubrac S, Elias PM, Feingold KR. Thematic review series: skin lipids. Peroxisome proliferator-activated receptors and liver X receptors in epidermal biology. J Lipid Res. 2008. 49:499–509.25. Lo Verme J, Fu J, Astarita G, La Rana G, Russo R, Calignano A, Piomelli D. The nuclear receptor peroxisome proliferator-activated receptor-alpha mediates the anti-inflammatory actions of palmitoylethanolamide. Mol Pharmacol. 2005. 67:15–19.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Adverse Effect of Topical Steroids
- Proper use of topical corticosteroids
- A Survey of Physician’s Awareness Regarding the Prescription and Side Effects of Topical Steroids
- Preventive Effects of Multi-Lamellar Emulsion on Low Potency Topical Steroid Induced Local Adverse Effect
- A Survey of the Awareness, Knowledge, and Behavior of Topical Steroid Use in Dermatologic Outpatients of the University Hospital