Allergy Asthma Immunol Res.
2011 Apr;3(2):89-95. 10.4168/aair.2011.3.2.89.
Long-term Efficacy of Intravenous Immunoglobulin Therapy for Moderate to Severe Childhood Atopic Dermatitis
- Affiliations
-
- 1Department of Pediatrics, Hanyang University College of Medicine, Seoul, Korea. jaewonoh@hanyang.ac.kr
- KMID: 2130359
- DOI: http://doi.org/10.4168/aair.2011.3.2.89
Abstract
- PURPOSE
The present study investigates the long-term effects of intravenous immunoglobulin (IVIg) therapy for the treatment of moderate to severe childhood atopic dermatitis (AD). Previous research indicates that IVIg can treat severe AD; however, the effectiveness of IVIg has not been confirmed in prospective, blinded clinical trials.
METHODS
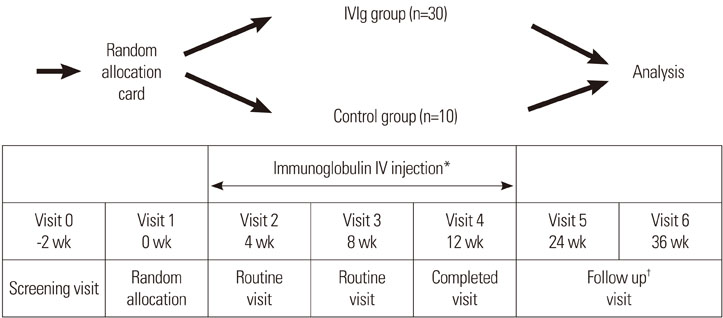
Forty eligible children with moderate to severe AD, as defined by the criteria of Hanifin and Rajka, were enrolled in a randomized, placebo-controlled study. After the completion of an initial screening visit (V0), the patients were randomly allocated into therapy (n=30) and control (n=10) groups (V1). Thirty children were each treated with three injections of 2.0 g/kg IVIg at 1-month intervals over a 12-week period. Ten children were treated with placebo. Assessments were conducted after each injection (V2, V3, and V4) and at 3 (V5) and 6 months (V6) after completed treatment.
RESULTS
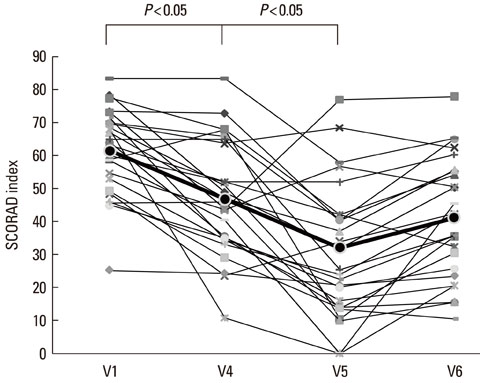
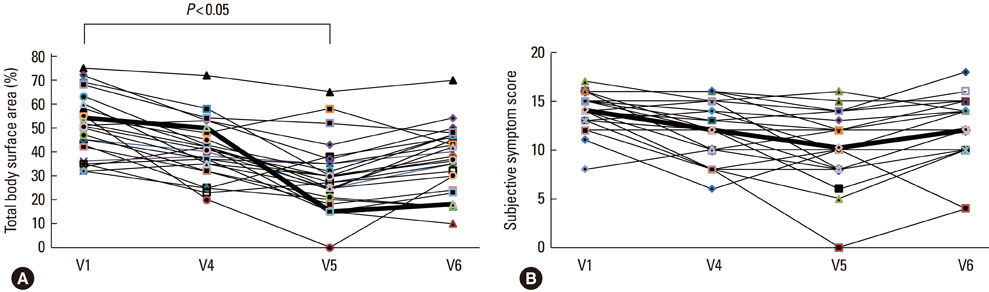
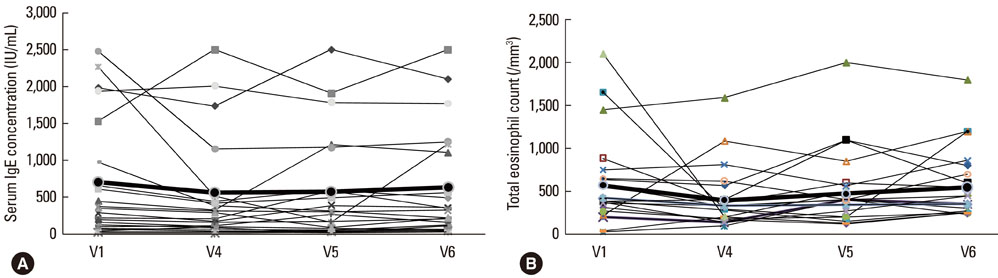
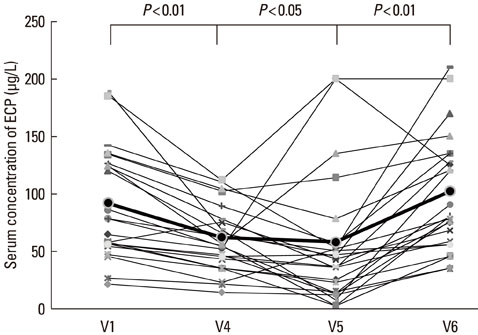
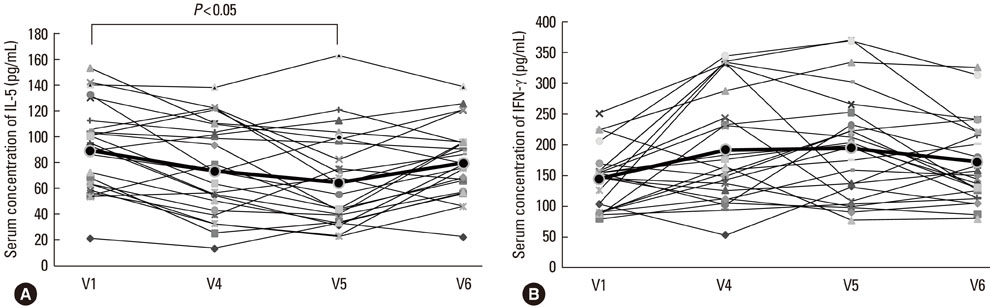
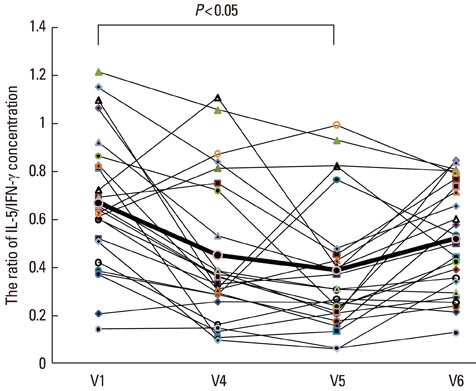
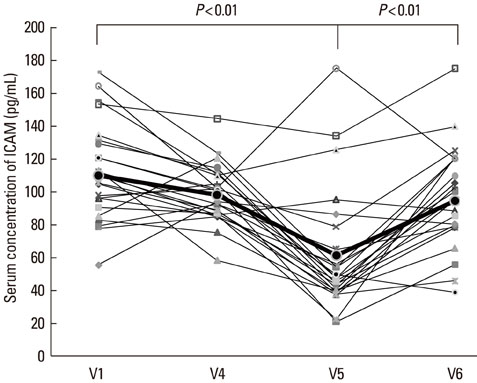
The disease severity index was significantly decreased at V5 compared with the value at V1 (P<0.05). There were no significant changes in the total IgE level or total eosinophil count in peripheral blood at the last injection (V4) compared with the value at V1. The interleukin (IL)-5/interferon (IFN)-gamma ratio was assessed in T-helper 1 (Th1) and Th2 cells. The ratio significantly decreased between V1 and V5, after which it increased, such that the ratio at V6 was not significantly different from that at V1. Compared with the level at V1, the intercellular cell adhesion molecule-1 level at V4 did not differ significantly, but the level at V5 was lower.
CONCLUSIONS
This study suggests that IVIg therapy may clinically improve AD in patients after 3 months of therapy, but the improvement may decline by 6 months after therapy.
MeSH Terms
Figure
Reference
-
1. Hanifin JM, Chan SC. Monocyte phosphodiesterase abnormalities and dysregulation of lymphocyte function in atopic dermatitis. J Invest Dermatol. 1995. 105:84S–88S.2. Sonenthal KR, Grammer LC, Patterson R. Do some patients with atopic dermatitis require long-term oral steroid therapy? J Allergy Clin Immunol. 1993. 91:971–973.3. Sowden JM, Berth-Jones J, Ross JS, Motley RJ, Marks R, Finlay AY, Salek MS, Graham-Brown RA, Allen BR, Camp RD. Double-blind, controlled, crossover study of cyclosporin in adults with severe refractory atopic dermatitis. Lancet. 1991. 338:137–140.4. Salek MS, Finlay AY, Luscombe DK, Allen BR, Berth-Jones J, Camp RD, Graham-Brown RA, Khan GK, Marks R, Motley RJ. Cyclosporin greatly improves the quality of life of adults with severe atopic dermatitis. A randomized, double-blind, placebo-controlled trial. Br J Dermatol. 1993. 129:422–430.5. Grossman RM, Chevret S, Abi-Rached J, Blanchet F, Dubertret L. Long-term safety of cyclosporine in the treatment of psoriasis. Arch Dermatol. 1996. 132:623–629.6. Kirby B, Owen CM, Blewitt RW, Yates VM. Cutaneous T-cell lymphoma developing in a patient on cyclosporin therapy. J Am Acad Dermatol. 2002. 47:S165–S167.7. Sewell WA, Jolles S. Immunomodulatory action of intravenous immunoglobulin. Immunology. 2002. 107:387–393.8. Spahn JD, Leung DY, Chan MT, Szefler SJ, Gelfand EW. Mechanisms of glucocorticoid reduction in asthmatic subjects treated with intravenous immunoglobulin. J Allergy Clin Immunol. 1999. 103:421–426.9. Samuelsson A, Towers TL, Ravetch JV. Anti-inflammatory activity of IVIG mediated through the inhibitory Fc receptor. Science. 2001. 291:484–486.10. Andersson J, Skansén-Saphir U, Sparrelid E, Andersson U. Intravenous immune globulin affects cytokine production in T lymphocytes and monocytes/macrophages. Clin Exp Immunol. 1996. 104:Suppl 1. 10–20.11. Bayry J, Lacroix-Desmazes S, Delignat S, Mouthon L, Weill B, Kazatchkine MD, Kaveri SV. Intravenous immunoglobulin abrogates dendritic cell differentiation induced by interferon-alpha present in serum from patients with systemic lupus erythematosus. Arthritis Rheum. 2003. 48:3497–3502.12. Bayry J, Lacroix-Desmazes S, Carbonneil C, Misra N, Donkova V, Pashov A, Chevailler A, Mouthon L, Weill B, Bruneval P, Kazatchkine MD, Kaveri SV. Inhibition of maturation and function of dendritic cells by intravenous immunoglobulin. Blood. 2003. 101:758–765.13. Jolles S, Sewell C, Webster D, Ryan A, Heelan B, Waite A, Rustin M. Adjunctive high-dose intravenous immunoglobulin treatment for resistant atopic dermatitis: efficacy and effects on intracellular cytokine levels and CD4 counts. Acta Derm Venereol. 2003. 83:433–437.14. Jolles S, Hughes J, Rustin M. The treatment of atopic dermatitis with adjunctive high-dose intravenous immunoglobulin: a report of three patients and review of the literature. Br J Dermatol. 2000. 142:551–554.15. Kimata H. High dose gammaglobulin treatment for atopic dermatitis. Arch Dis Child. 1994. 70:335–336.16. Wakim M, Alazard M, Yajima A, Speights D, Saxon A, Stiehm ER. High dose intravenous immunoglobulin in atopic dermatitis and hyper-IgE syndrome. Ann Allergy Asthma Immunol. 1998. 81:153–158.17. Paul C, Lahfa M, Bachelez H, Chevret S, Dubertret L. A randomized controlled evaluator-blinded trial of intravenous immunoglobulin in adults with severe atopic dermatitis. Br J Dermatol. 2002. 147:518–522.18. Hanifin JM, Rajka G. Diagnostic features of atopic dermatitis. Acta Derm Venereol Suppl. 1980. 114:146–148.19. Prins C, Gelfand EW, French LE. Intravenous immunoglobulin: properties, mode of action and practical use in dermatology. Acta Derm Venereol. 2007. 87:206–218.20. Kowalzick L, Kleinheinz A, Neuber K, Weichenthal M, Köhler I, Ring J. Elevated serum levels of soluble adhesion molecules ICAM-1 and ELAM-1 in patients with severe atopic eczema and influence of UVA1 treatment. Dermatology. 1995. 190:14–18.21. Wakita H, Sakamoto T, Tokura Y, Takigawa M. E-selectin and vascular cell adhesion molecule-1 as critical adhesion molecules for infiltration of T lymphocytes and eosinophils in atopic dermatitis. J Cutan Pathol. 1994. 21:33–39.22. Halmerbauer G, Frischer T, Koller DY. Monitoring of disease activity by measurement of inflammatory markers in atopic dermatitis in childhood. Allergy. 1997. 52:765–769.23. Grewe M, Bruijnzeel-Koomen CA, Schöpf E, Thepen T, Langeveld-Wildschut AG, Ruzicka T, Krutmann J. A role for Th1 and Th2 cells in the immunopathogenesis of atopic dermatitis. Immunol Today. 1998. 19:359–361.24. Chan S, Henderson WR Jr, Li SH, Hanifin JM. Prostaglandin E2 control of T cell cytokine production is functionally related to the reduced lymphocyte proliferation in atopic dermatitis. J Allergy Clin Immunol. 1996. 97:85–94.25. Thepen T, Langeveld-Wildschut EG, Bihari IC, van Wichen DF, van Reijsen FC, Mudde GC, Bruijnzeel-Koomen CA. Biphasic response against aeroallergen in atopic dermatitis showing a switch from an initial TH2 response to a TH1 response in situ: an immunocytochemical study. J Allergy Clin Immunol. 1996. 97:828–837.26. Hamid Q, Naseer T, Minshall EM, Song YL, Boguniewicz M, Leung DY. In vivo expression of IL-12 and IL-13 in atopic dermatitis. J Allergy Clin Immunol. 1996. 98:225–231.27. Gelfand EW. Differences between IGIV products: impact on clinical outcome. Int Immunopharmacol. 2006. 6:592–599.28. Nydegger UE, Sturzenegger M. Adverse effects of intravenous immunoglobulin therapy. Drug Saf. 1999. 21:171–185.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Long-term Effect of Recombinant Interferon-Gamma on Moderate and Severe Atopic Dermatitis in Childhood
- The Clinical Effect of Topical Diphenylcyclopropenone (DPCP) Therapy in the Treatment of Atopic Dermatitis in Children and Adolescents
- Efficacy of Intravenous Immunoglobulin Therapy in Childhood Atopic Dermatitis
- Clinical Investigation of Children with Severe Atopic Dermatitis
- Autologous Immunoglobulin Therapy in Patients With Severe Recalcitrant Atopic Dermatitis: Long-Term Changes of Clinical Severity and Laboratory Parameters