Allergy Asthma Immunol Res.
2015 Nov;7(6):599-604. 10.4168/aair.2015.7.6.599.
Attenuated Allergenic Activity of Ovomucoid After Electrolysis
- Affiliations
-
- 1Department of Pediatrics, Kumamoto Regional Medical Center, Kumamoto City, Kumamoto, Japan. matsumoto@a.asospahp.jp
- 2Department of Pediatrics, Graduate School of Medical Sciences, Kumamoto University, Kumamoto City, Kumamoto, Japan.
- 3Department of Child Development, Kumamoto University Hospital, Kumamoto City, Kumamoto, Japan.
- 4Pediatric Division, Aso Spa Hospital, Aso City, Kumamoto, Japan.
- KMID: 2130267
- DOI: http://doi.org/10.4168/aair.2015.7.6.599
Abstract
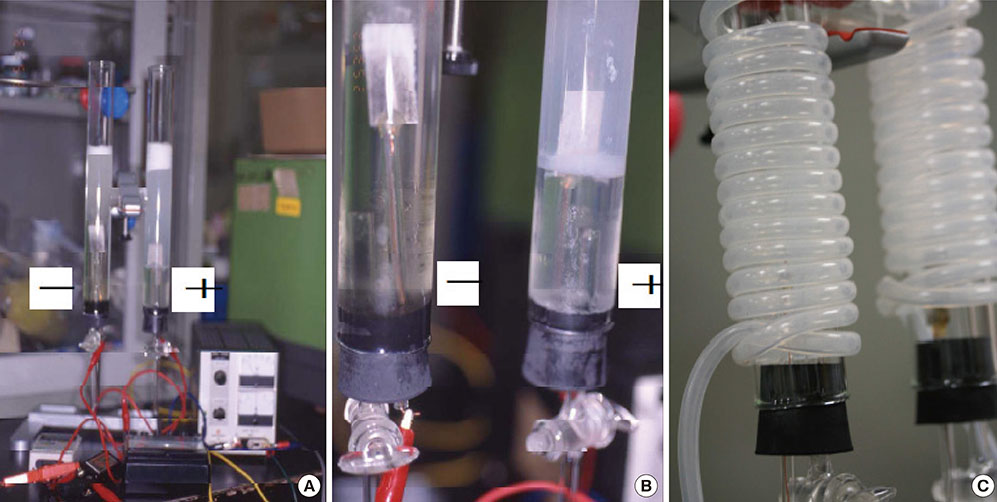
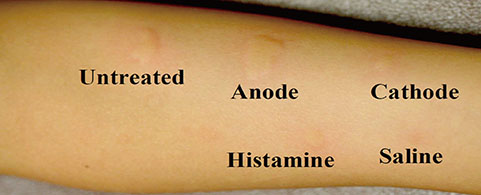
- Ovomucoid (OMC) is the most prominent allergen causing hen's egg allergy, containing disulfide (S-S) bonds that may be responsible for its allergic action. As S-S bonds may be reduced during electrolysis, this study was undertaken to evaluate modulation of the allergic action of OMC after electrolysis. Electrolysis was carried out for 1% OMC containing 1% sodium chloride for 30 minutes with a voltage difference of 90 V, 0.23 A (30 mA/cm2). Protein assays, amino acid measurement, and mass spectrometry in untreated OMC and OMC on both the anode and cathode sides after electrolysis were performed. Moreover, 21 patients with IgE-mediated hen's egg allergy were evaluated by using the skin prick test (SPT) for untreated OMC and OMC after electrolysis. The allergic action of OMC was reduced after electrolysis on both the anode and cathode sides when evaluated by the SPT. The modifications of OMC on electrolysis caused the loss of 2 distinct peptide fragments (57E-63K and 123H-128R) as seen on matrix-associated laser desorption/ionization time-of-flight mass spectrometry. The total free SH groups in OMC were increased on the cathode side. Although the regions of S-S broken bonds were not determined in this study, the change in S-S bonds in OMC on both the anode and cathode sides may reduce the allergenic activity.
Keyword
MeSH Terms
Figure
Reference
-
1. Ebisawa M, Sugizaki C. Prevalence of pediatric allergic diseases in the first 5 years of life. J Allergy Clin Immunol. 2008; 121:S237.2. Noda R. Prevalence of food allergy in nursery school (nationwide survey). Jpn J Food Allergy. 2010; 10:5–9.3. Bernhisel-Broadbent J, Dintzis HM, Dintzis RZ, Sampson HA. Allergenicity and antigenicity of chicken egg ovomucoid (Gal d III) compared with ovalbumin (Gal d I) in children with egg allergy and in mice. J Allergy Clin Immunol. 1994; 93:1047–1059.4. Kovacs-Nolan J, Zhang JW, Hayakawa S, Mine Y. Immunochemical and structural analysis of pepsin-digested egg white ovomucoid. J Agric Food Chem. 2000; 48:6261–6266.5. Cooke SK, Sampson HA. Allergenic properties of ovomucoid in man. J Immunol. 1997; 159:2026–2032.6. Mine Y, Sasaki E, Zhang JW. Reduction of antigenicity and allergenicity of genetically modified egg white allergen, ovomucoid third domain. Biochem Biophys Res Commun. 2003; 302:133–137.7. Urisu A, Ando H, Morita Y, Wada E, Yasaki T, Yamada K, et al. Allergenic activity of heated and ovomucoid-depleted egg white. J Allergy Clin Immunol. 1997; 100:171–176.8. Kato Y, Oozawa E, Matsuda T. Decrease in antigenic and allergenic potentials of ovomucoid by heating in the presence of wheat flour: dependence on wheat variety and intermolecular disulfide bridges. J Agric Food Chem. 2001; 49:3661–3665.9. Matsumoto T. Mitigation of the allergenic activity of beta-lactoglobulin by electrolysis. Pediatr Allergy Immunol. 2011; 22:235–242.10. Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951; 193:265–275.11. Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959; 82:70–77.12. Gevaert K, Verschelde JL, Puype M, Van Damme J, Goethals M, De Boeck S, et al. Structural analysis and identification of gel-purified proteins, available in the femtomole range, using a novel computer program for peptide sequence assignment, by matrix-assisted laser desorption ionization-reflectron time-of-flight-mass spectrometry. Electrophoresis. 1996; 17:918–924.13. Kato I, Schrode J, Kohr WJ, Laskowski M Jr. Chicken ovomucoid: determination of its amino acid sequence, determination of the trypsin reactive site, and preparation of all three of its domains. Biochemistry. 1987; 26:193–201.14. A Hosono Y Ooi R Nakamura . Hypoallergenic egg white and a method of manufacturing hypoallergenic egg white. Japanese patent. P2004-261154A. 2004. Sep. 24.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Influence of the Presence of Wheat Flour on the Antigenic Activities of Egg White Proteins
- Antimicrobial effect of slightly acidic electrolyzed water on oral microorganisms
- Is There Any Necessity to Prescribe Consumption of Walnuts Cooked by Different Processing Techniques to Patients With Walnut Allergy?
- Electrolysis of physiological salt solution generates a factor that relaxes vascular smooth muscle
- Histopathologic Findings of Rabbit Cilia after Application of Radio Frequency Wave