Allergy Asthma Immunol Res.
2015 Nov;7(6):583-589. 10.4168/aair.2015.7.6.583.
Blocking of Histamine Release and IgE Binding to FcepsilonRI on Human Basophils by Antibodies Produced in Camels
- Affiliations
-
- 1Department of Biological Sciences-Yarmouk University, Irbid, Jordan. khaled@monojo.com.jo
- 2Jordan Company for Antibody Production (MONOJO), Amman, Jordan.
- 3Department of Biotechnology and Genetic Engineering, Philadelphia University, Amman, Jordan.
- 4World Islamic Science and Education University (WISE), Amman, Jordan.
- KMID: 2130265
- DOI: http://doi.org/10.4168/aair.2015.7.6.583
Abstract
- PURPOSE
The production of camel heavy-chain antihuman IgE (huIgE) that has the potential to block IgE-FcepsilonRI interaction and histamine release by basophils.
METHODS
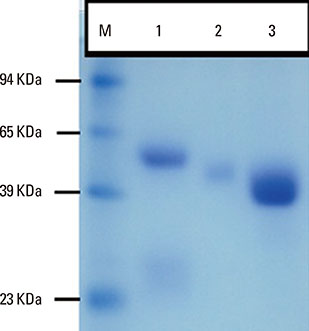
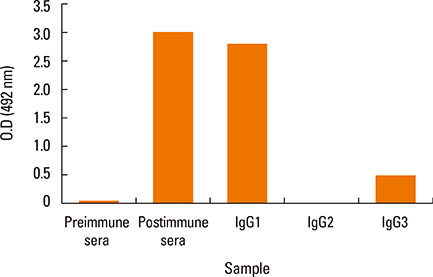
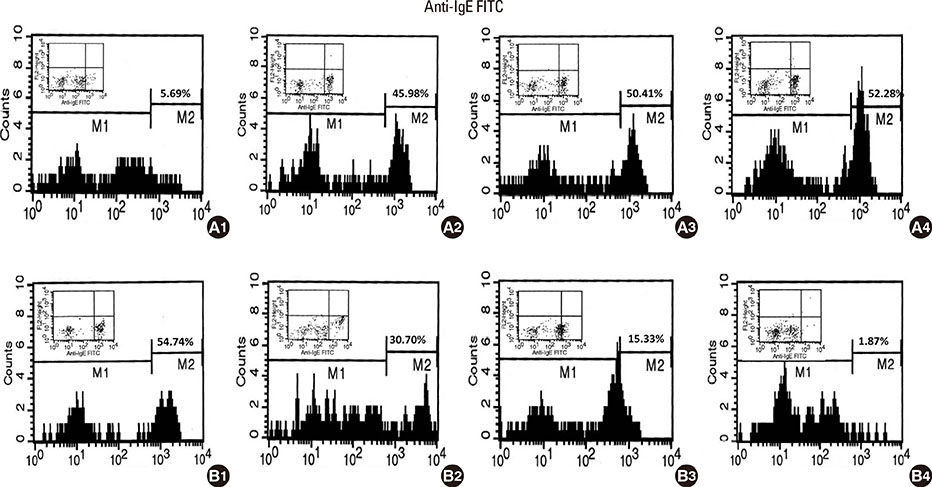
Camels were immunized with a synthetic loop peptide (SLP) designed in a multiple antigen peptide system (MAPS) forming SLP-MAPS immunogen. Camel polyclonal antibodies (PCAs) were produced, purified, characterized using Protein A & G, ELISA, and SDS-PAGE, and tested for their potency to block passive sensitization and histamine release of human basophils using flow cytometry (FCM) and ELISA, respectively.
RESULTS
FCM data indicated that camel conventional (IgG1) and heavy chain antibodies (HCAbs; IgG2, and IgG3) had blocking activities of 43.9%, 72%, and 96.6%, respectively. Moreover, both IgG2 and IgG3 achieved remarkable inhibition rates of 93.98% and 97.05% in histamine release, respectively, whereas the IgG1inhibiting activity was 60.05%.
CONCLUSIONS
Camel PCAs produced against SLP-MAPS were capable of blocking the IgE-receptor interaction and the release of histamine by basophils with superiority to HCAbs. These findings may pave the way toward the possible use of camel anti-huIgE HCAbs as blocking antibodies in the treatment of IgE-mediated allergy and asthma.
Keyword
MeSH Terms
-
Antibodies*
Antibodies, Blocking
Asthma
Basophils*
Camels*
Electrophoresis, Polyacrylamide Gel
Enzyme-Linked Immunosorbent Assay
Flow Cytometry
Histamine Release*
Histamine*
Humans*
Hypersensitivity
Immunoglobulin E*
Immunoglobulin G
Passive Cutaneous Anaphylaxis
Staphylococcal Protein A
Antibodies
Antibodies, Blocking
Histamine
Immunoglobulin E
Immunoglobulin G
Staphylococcal Protein A
Figure
Reference
-
1. Braman SS. The global burden of asthma. Chest. 2006; 130:4S–12S.2. Myers TR, Tomasio L. Asthma: 2015 and beyond. Respir Care. 2011; 56:1389–1407.3. Masoli M, Fabian D, Holt S, Beasley R. Global Initiative for Asthma (GINA) Program. The global burden of asthma: executive summary of the GINA Dissemination Committee report. Allergy. 2004; 59:469–478.4. Gould HJ, Sutton BJ. IgE in allergy and asthma today. Nat Rev Immunol. 2008; 8:205–217.5. D'Amato G. Role of anti-IgE monoclonal antibody (omalizumab) in the treatment of bronchial asthma and allergic respiratory diseases. Eur J Pharmacol. 2006; 533:302–307.6. Peng Z. Vaccines targeting IgE in the treatment of asthma and allergy. Hum Vaccin. 2009; 5:302–309.7. Verdoliva A, Rossi M. IgE-mediated disorders: current therapeutics and new strategies involving synthetic peptides. Antiinflamm Antiallergy Agents Med Chem. 2008; 7:252–263.8. Chang TW. The pharmacological basis of anti-IgE therapy. Nat Biotechnol. 2000; 18:157–162.9. Hamelmann E. The rationale for treating allergic asthma with anti-IgE. Eur Respir Rev. 2007; 16:61–66.10. Hamers-Casterman C, Atarhouch T, Muyldermans S, Robinson G, Hamers C, Songa EB, et al. Naturally occurring antibodies devoid of light chains. Nature. 1993; 363:446–448.11. Muyldermans S. Single domain camel antibodies: current status. J Biotechnol. 2001; 74:277–302.12. Muyldermans S, Atarhouch T, Saldanha J, Barbosa JA, Hamers R. Sequence and structure of VH domain from naturally occurring camel heavy chain immunoglobulins lacking light chains. Protein Eng. 1994; 7:1129–1135.13. Wesolowski J, Alzogaray V, Reyelt J, Unger M, Juarez K, Urrutia M, et al. Single domain antibodies: promising experimental and therapeutic tools in infection and immunity. Med Microbiol Immunol. 2009; 198:157–174.14. Arbabi Ghahroudi M, Desmyter A, Wyns L, Hamers R, Muyldermans S. Selection and identification of single domain antibody fragments from camel heavy-chain antibodies. FEBS Lett. 1997; 414:521–526.15. Franco EJ, Sonneson GJ, DeLegge TJ, Hofstetter H, Horn JR, Hofstetter O. Production and characterization of a genetically engineered anti-caffeine camelid antibody and its use in immunoaffinity chromatography. J Chromatogr B Analyt Technol Biomed Life Sci. 2010; 878:177–186.16. Muyldermans S, Baral TN, Retamozzo VC, De Baetselier P, De Genst E, Kinne J, et al. Camelid immunoglobulins and nanobody technology. Vet Immunol Immunopathol. 2009; 128:178–183.17. van de Laa T, Visser C, Holster M, López CG, Kreuning D, Sierkstra L, et al. Increased heterologous protein production by Saccharomyces cerevisiae growing on ethanol as sole carbon source. Biotechnol Bioeng. 2007; 96:483–494.18. Wang CY, Walfield AM, Fang X, Hammerberg B, Ye J, Li ML, et al. Synthetic IgE peptide vaccine for immunotherapy of allergy. Vaccine. 2003; 21:1580–1590.19. Tam JP. Synthetic peptide vaccine design: synthesis and properties of a high-density multiple antigenic peptide system. Proc Natl Acad Sci U S A. 1988; 85:5409–5413.20. Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976; 72:248–254.21. Pruzansky JJ, Grammer LC, Patterson R, Roberts M. Dissociation of IgE from receptors on human basophils. I. Enhanced passive sensitization for histamine release. J Immunol. 1983; 131:1949–1953.22. Rahbarizadeh F, Rasaee MJ, Forouzandeh M, Allameh A, Sarrami R, Nasiry H, et al. The production and characterization of novel heavy-chain antibodies against the tandem repeat region of MUC1 mucin. Immunol Invest. 2005; 34:431–452.23. Daley LP, Kutzler MA, Bennett BW, Smith MC, Glaser AL, Appleton JA. Effector functions of camelid heavy-chain antibodies in immunity to West Nile virus. Clin Vaccine Immunol. 2010; 17:239–246.24. Conrath KE, Lauwereys M, Galleni M, Matagne A, Frère JM, Kinne J, et al. β-Lactamase inhibitors derived from single-domain antibody fragments elicited in the camelidae. Antimicrob Agents Chemother. 2001; 45:2807–2812.25. Meddeb-Mouelhi F, Bouhaouala-Zahar B, Benlasfar Z, Hammadi M, Mejri T, Moslah M, et al. Immunized camel sera and derived immunoglobulin subclasses neutralizing Androctonus australis hector scorpion toxins. Toxicon. 2003; 42:785–791.26. Helm B, Kebo D, Vercelli D, Glovsky MM, Gould H, Ishizaka K, et al. Blocking of passive sensitization of human mast cells and basophil granulocytes with IgE antibodies by a recombinant human epsilon-chain fragment of 76 amino acids. Proc Natl Acad Sci U S A. 1989; 86:9465–9469.27. Wan D, Ludolf F, Alanine DG, Stretton O, Ali Ali E, Al-Barwary N, et al. Use of humanised rat basophilic leukaemia cell line RS-ATL8 for the assessment of allergenicity of Schistosoma mansoni proteins. PLoS Negl Trop Dis. 2014; 8:e3124.28. Sainte-Laudy J, Sabbah A, Vallon C, Guerin JC. Analysis of anti-IgE and allergen induced human basophil activation by flow cytometry. Comparison with histamine release. Inflamm Res. 1998; 47:401–408.29. Smolarek D, Bertrand O, Czerwinski M. Variable fragments of heavy chain antibodies (VHHs): a new magic bullet molecule of medicine? Postepy Hig Med Dosw (Online). 2012; 66:348–358.30. Vincke C, Loris R, Saerens D, Martinez-Rodriguez S, Muyldermans S, Conrath K. General strategy to humanize a camelid single-domain antibody and identification of a universal humanized nanobody scaffold. J Biol Chem. 2009; 284:3273–3284.31. Harmsen MM, De Haard HJ. Properties, production, and applications of camelid single-domain antibody fragments. Appl Microbiol Biotechnol. 2007; 77:13–22.32. Dodig S, Richter D, Cepelak I, Benko B. Anti-IgE therapy with omalizumab in asthma and allergic rhinitis. Acta Pharm. 2005; 55:123–138.33. Milgrom H, Fick RB Jr, Su JQ, Reimann JD, Bush RK, Watrous ML, et al. Treatment of allergic asthma with monoclonal anti-IgE antibody. rhuMAb-E25 Study Group. N Engl J Med. 1999; 341:1966–1973.34. Lanier B, Bridges T, Kulus M, Taylor AF, Berhane I, Vidaurre CF. Omalizumab for the treatment of exacerbations in children with inadequately controlled allergic (IgE-mediated) asthma. J Allergy Clin Immunol. 2009; 124:1210–1216.35. Slavin RG, Ferioli C, Tannenbaum SJ, Martin C, Blogg M, Lowe PJ. Asthma symptom re-emergence after omalizumab withdrawal correlates well with increasing IgE and decreasing pharmacokinetic concentrations. J Allergy Clin Immunol. 2009; 123:107–113.e3.36. Davydov L. Omalizumab (Xolair) for treatment of asthma. Am Fam Physician. 2005; 71:341–342.37. Dumoulin M, Conrath K, Van Meirhaeghe A, Meersman F, Heremans K, Frenken LG, et al. Single-domain antibody fragments with high conformational stability. Protein Sci. 2002; 11:500–515.38. Harmsen MM, van Solt CB, van Zijderveld-van Bemmel AM, Niewold TA, van Zijderveld FG. Selection and optimization of proteolytically stable llama single-domain antibody fragments for oral immunotherapy. Appl Microbiol Biotechnol. 2006; 72:544–551.39. van der Linden RH, Frenken LG, de Geus B, Harmsen MM, Ruuls RC, Stok W, et al. Comparison of physical chemical properties of llama VHH antibody fragments and mouse monoclonal antibodies. Biochim Biophys Acta. 1999; 1431:37–46.40. Hussack G, Hirama T, Ding W, Mackenzie R, Tanha J. Engineered single-domain antibodies with high protease resistance and thermal stability. PLoS One. 2011; 6:e28218.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Effects of Nonspecific IgE and IgG Antibodies on Basophil Histamine Release mediated by Specific IgE Antibodies
- The Influence of IgE on Cultured Human Mast Cells
- Linkage between gene marker of chromosome 11q13 and IgE-receptor mediated basophil histamine release
- Basophil histamine releasability in children with atopic asthma
- Basophil Histamine Releasability in Asthmatic Children