J Korean Med Sci.
2014 Aug;29(8):1138-1144. 10.3346/jkms.2014.29.8.1138.
Neuropathic Pain Model of Peripheral Neuropathies Mediated by Mutations of Glycyl-tRNA Synthetase
- Affiliations
-
- 1Department of Anatomy and Neurobiology, School of Medicine, Kyung Hee University, Seoul, Korea. ybhuh@khu.ac.kr
- KMID: 2129611
- DOI: http://doi.org/10.3346/jkms.2014.29.8.1138
Abstract
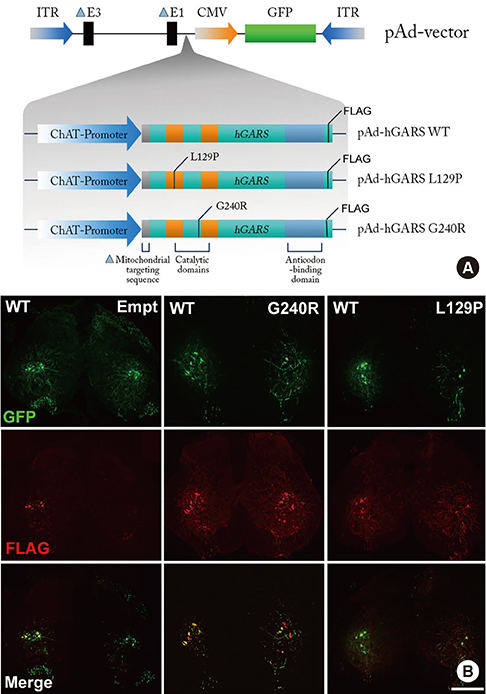
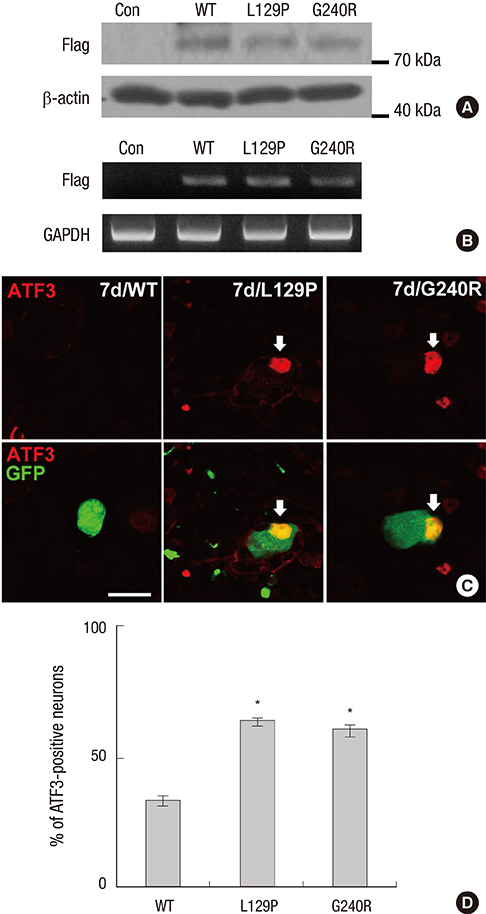
- Charcot-Marie-Tooth disease (CMT) is the most common inherited motor and sensory neuropathy. Previous studies have found that, according to CMT patients, neuropathic pain is an occasional symptom of CMT. However, neuropathic pain is not considered to be a significant symptom associated with CMT and, as a result, no studies have investigated the pathophysiology underlying neuropathic pain in this disorder. Thus, the first animal model of neuropathic pain was developed by our laboratory using an adenovirus vector system to study neuropathic pain in CMT. To this end, glycyl-tRNA synthetase (GARS) fusion proteins with a FLAG-tag (wild type [WT], L129P and G240R mutants) were expressed in spinal cord and dorsal root ganglion (DRG) neurons using adenovirus vectors. It is known that GARS mutants induce GARS axonopathies, including CMT type 2D (CMT2D) and distal spinal muscular atrophy type V (dSMA-V). Additionally, the morphological phenotypes of neuropathic pain in this animal model of GARS-induced pain were assessed using several possible markers of pain (Iba1, pERK1/2) or a marker of injured neurons (ATF3). These results suggest that this animal model of CMT using an adenovirus may provide information regarding CMT as well as a useful strategy for the treatment of neuropathic pain.
Keyword
MeSH Terms
Figure
Reference
-
1. Baron R. Mechanisms of disease: neuropathic pain: a clinical perspective. Nat Clin Pract Neurol. 2006; 2:95–106.2. Woolf CJ, Salter MW. Neuronal plasticity: increasing the gain in pain. Science. 2000; 288:1765–1769.3. Costigan M, Scholz J, Woolf CJ. Neuropathic pain: a maladaptive response of the nervous system to damage. Annu Rev Neurosci. 2009; 32:1–32.4. Tsuda M, Shigemoto-Mogami Y, Koizumi S, Mizokoshi A, Kohsaka S, Salter MW, Inoue K. P2X4 receptors induced in spinal microglia gate tactile allodynia after nerve injury. Nature. 2003; 424:778–783.5. Tsuda M, Inoue K, Salter MW. Neuropathic pain and spinal microglia: a big problem from molecules in "small" glia. Trends Neurosci. 2005; 28:101–107.6. Emery AE. Population frequencies of inherited neuromuscular diseases: a world survey. Neuromuscul Disord. 1991; 1:19–29.7. Carter GT, Jensen MP, Galer BS, Kraft GH, Crabtree LD, Beardsley RM, Abresch RT, Bird TD. Neuropathic pain in Charcot-Marie-Tooth disease. Arch Phys Med Rehabil. 1998; 79:1560–1564.8. Pazzaglia C, Vollono C, Ferraro D, Virdis D, Lupi V, Le Pera D, Tonali P, Padua L, Valeriani M. Mechanisms of neuropathic pain in patients with Charcot-Marie-Tooth 1 A: a laser-evoked potential study. Pain. 2010; 149:379–385.9. Ribiere C, Bernardin M, Sacconi S, Delmont E, Fournier-Mehouas M, Rauscent H, Benchortane M, Staccini P, Lantéri-Minet M, Desnuelle C. Pain assessment in Charcot-Marie-Tooth (CMT) disease. Ann Phys Rehabil Med. 2012; 55:160–173.10. Antonellis A, Ellsworth RE, Sambuughin N, Puls I, Abel A, Lee-Lin SQ, Jordanova A, Kremensky I, Christodoulou K, Middleton LT, et al. Glycyl tRNA synthetase mutations in Charcot-Marie-Tooth disease type 2D and distal spinal muscular atrophy type V. Am J Hum Genet. 2003; 72:1293–1299.11. Sambuughin N, Sivakumar K, Selenge B, Lee HS, Friedlich D, Baasanjav D, Dalakas MC, Goldfarb LG. Autosomal dominant distal spinal muscular atrophy type V (dSMA-V) and Charcot-Marie-Tooth disease type 2D (CMT2D) segregate within a single large kindred and map to a refined region on chromosome 7p15. J Neurol Sci. 1998; 161:23–28.12. Ionasescu V, Searby C, Sheffield VC, Roklina T, Nishimura D, Ionasescu R. Autosomal dominant Charcot-Marie-Tooth axonal neuropathy mapped on chromosome 7p (CMT2D). Hum Mol Genet. 1996; 5:1373–1375.13. Motley WW, Talbot K, Fischbeck KH. GARS axonopathy: not every neuron's cup of tRNA. Trends Neurosci. 2010; 33:59–66.14. Bráz JM, Basbaum AI. Differential ATF3 expression in dorsal root ganglion neurons reveals the profile of primary afferents engaged by diverse noxious chemical stimuli. Pain. 2010; 150:290–301.15. Ivanavicius SP, Ball AD, Heapy CG, Westwood FR, Murray F, Read SJ. Structural pathology in a rodent model of osteoarthritis is associated with neuropathic pain: increased expression of ATF-3 and pharmacological characterisation. Pain. 2007; 128:272–282.16. Ma W, Quirion R. Partial sciatic nerve ligation induces increase in the phosphorylation of extracellular signal-regulated kinase (ERK) and c-Jun N-terminal kinase (JNK) in astrocytes in the lumbar spinal dorsal horn and the gracile nucleus. Pain. 2002; 99:175–184.17. Maeda M, Tsuda M, Tozaki-Saitoh H, Inoue K, Kiyama H. Nerve injury-activated microglia engulf myelinated axons in a P2Y12 signaling-dependent manner in the dorsal horn. Glia. 2010; 58:1838–1846.18. Misawa H, Ishii K, Deguchi T. Gene expression of mouse choline acetyltransferase. Alternative splicing and identification of a highly active promoter region. J Biol Chem. 1992; 267:20392–20399.19. Lönnerberg P, Schoenherr CJ, Anderson DJ, Ibáñez CF. Cell type-specific regulation of choline acetyltransferase gene expression: role of the neuron-restrictive silencer element and cholinergic-specific enhancer sequences. J Biol Chem. 1996; 271:33358–33365.20. Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994; 53:55–63.21. Christodoulou K, Kyriakides T, Hristova AH, Georgiou DM, Kalaydjieva L, Yshpekova B, Ivanova T, Weber JL, Middleton LT. Mapping of a distal form of spinal muscular atrophy with upper limb predominance to chromosome 7p. Hum Mol Genet. 1995; 4:1629–1632.22. Marchand F, Perretti M, McMahon SB. Role of the immune system in chronic pain. Nat Rev Neurosci. 2005; 6:521–532.23. Watkins LR, Maier SF. Glia: a novel drug discovery target for clinical pain. Nat Rev Drug Discov. 2003; 2:973–985.24. Zhuang ZY, Gerner P, Woolf CJ, Ji RR. ERK is sequentially activated in neurons, microglia, and astrocytes by spinal nerve ligation and contributes to mechanical allodynia in this neuropathic pain model. Pain. 2005; 114:149–159.25. Ma W, Quirion R. Partial sciatic nerve ligation induces increase in the phosphorylation of extracellular signal-regulated kinase (ERK) and c-Jun N-terminal kinase (JNK) in astrocytes in the lumbar spinal dorsal horn and the gracile nucleus. Pain. 2002; 99:175–184.26. Tsujino H, Kondo E, Fukuoka T, Dai Y, Tokunaga A, Miki K, Yonenobu K, Ochi T, Noguchi K. Activating transcription factor 3 (ATF3) induction by axotomy in sensory and motoneurons: a novel neuronal marker of nerve injury. Mol Cell Neurosci. 2000; 15:170–182.27. Bellier JP, Kimura H. Acetylcholine synthesis by choline acetyltransferase of a peripheral type as demonstrated in adult rat dorsal root ganglion. J Neurochem. 2007; 101:1607–1618.28. Matsumoto M, Xie W, Inoue M, Ueda H. Evidence for the tonic inhibition of spinal pain by nicotinic cholinergic transmission through primary afferents. Mol Pain. 2007; 3:41.29. Nangle LA, Zhang W, Xie W, Yang XL, Schimmel P. Charcot-Marie-Tooth disease-associated mutant tRNA synthetases linked to altered dimer interface and neurite distribution defect. Proc Natl Acad Sci U S A. 2007; 104:11239–11244.30. Seo AJ, Shin YH, Lee SJ, Kim D, Park BS, Kim S, Choi KH, Jeong NY, Park C, Jang JY, et al. A novel adenoviral vector-mediated mouse model of Charcot-Marie-Tooth type 2D (CMT2D). J Mol Histol. 2014; 45:121–128.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Peripheral Neuropathy
- New Paradigm for the Neuropathic Pain
- Molecular Analysis of Isoleucyl-tRNA Synthetase Mutations in Clinical Isolates of Methicillin-Resistant Staphylococcus aureus with Low-Level Mupirocin Resistance
- Treatment of peripheral neuropathy: a multidisciplinary approach is necessary
- Thermography in Peripheral Neuropathic Pain after Peripheral Nerve Injuries