J Korean Med Sci.
2014 Aug;29(8):1108-1112. 10.3346/jkms.2014.29.8.1108.
Comparison of Korean COPD Guideline and GOLD Initiative Report in Term of Acute Exacerbation: A Validation Study for Korean COPD Guideline
- Affiliations
-
- 1Department of Pulmonary, Allergy and Critical Care Medicine, Hallym University Medical Center, Hallym University College of Medicine, Anyang, Korea. pulmoks@hallym.ac.kr
- 2Department of Pulmonary and Critical Care Medicine, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- 3Department of Internal Medicine, CHA Bundang Medical Center, CHA University, Seongnam, Korea.
- 4Department of Internal Medicine, Hanyang University Guri Hospital, Hanyang University College of Medicine, Guri, Korea.
- 5Department of Internal Medicine, Konkuk University College of Medicine, Seoul, Korea.
- 6Department of Internal Medicine, Yeouido St. Marry's Hosital, The Catholic University of Korea College of Medicine, Seoul, Korea.
- 7Department of Internal Medicine, St. Mary's Hospital, The Catholic University of Korea College of Medicine, Seoul, Korea.
- 8Department of Internal Medicine, Seoul Metropolitan Government-Seoul National University Boramae Medical Center, Seoul, Korea.
- 9Department of Internal Medicine,Yeungnam University College of Medicine, Daegu, Korea.
- 10Department of Internal Medicine, Korea University Anam Hospital, Korea University College of Medicine, Seoul, Korea.
- KMID: 2129606
- DOI: http://doi.org/10.3346/jkms.2014.29.8.1108
Abstract
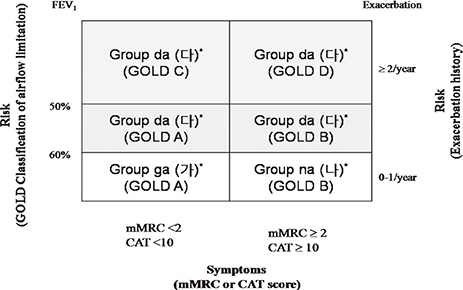
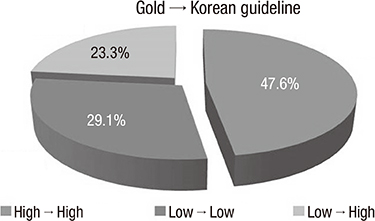
- The purpose of this study was to compare the Korean COPD guideline to GOLD consensus report in terms of acute exacerbation. A total of 361 patients were enrolled in this study, and 16.9% of them experienced acute exacerbation during the follow-up. A total of 6.3% of patients in GOLD A, 9.5% in GOLD B, 7.7% in GOLD C and 17.0% of GOLD D experienced exacerbation during the first year of follow-up, respectively (P=0.09). There was no one who experienced exacerbation during the first year of follow-up in the Korean group 'ga'. The 12-month exacerbation rates of Korean group 'na' and 'da' were 4.5% and 16.0%, respectively (P<0.001). We explore the experience of exacerbation in patients with change of their risk group after applying Korean COPD guideline. A total of 16.0% of the patients who were reclassified from GOLD A to Korean group 'da' experienced acute exacerbation,and 15.3% from GOLD B to Korean group 'da' experienced acute exacerbation. In summary, the Korean COPD guideline is useful to differentiate the high risk from low risk for exacerbation in terms of spirometry. This indicates that application of Korean COPD guideline is appropriate to treat Korean COPD patients.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Efficacy and Safety of Roflumilast in Korean Patients with COPD
Jae Seung Lee, Yoon Ki Hong, Tae Sun Park, Sei Won Lee, Yeon-Mok Oh, Sang-Do Lee
Yonsei Med J. 2016;57(4):928-935. doi: 10.3349/ymj.2016.57.4.928.
Reference
-
1. Global Initiative for Chronic Obstructive Lung Disease. Publication Number 2701. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: NHLBI/WHO Workshop Report, National Institutes of Health. National Heart, Lung, and Blood Institute;2001.2. Hurst JR, Vestbo J, Anzueto A, Locantore N, Müllerova H, Tal-Singer R, Miller B, Lomas DA, Agusti A, Macnee W, et al. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med. 2010; 363:1128–1138.3. Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management and Prevent of chronic obstructive pulmonary disease, updated 2014. accessed on 5 February 2014. Available at http://www.goldcopd.org.4. Han MK, Muellerova H, Curran-Everett D, Dransfield MT, Washko GR, Regan EA, Bowler RP, Beaty TH, Hokanson JE, Lynch DA, et al. GOLD 2011 disease severity classification in COPDGene: a prospective cohort study. Lancet Respir Med. 2013; 1:43–50.5. Lange P, Marott JL, Vestbo J, Olsen KR, Ingebrigtsen TS, Dahl M, Nordestgaard BG. Prediction of the clinical course of chronic obstructive pulmonary disease, using the new GOLD classification: a study of the general population. Am J Respir Crit Care Med. 2012; 186:975–981.6. Soriano JB, Alfageme I, Almagro P, Casanova C, Esteban C, Soler-Cataluña JJ, de Torres JP, Martinez-Camblor P, Miravitlles M, Celli BR, et al. Distribution and prognostic validity of the new Global Initiative for Chronic Obstructive Lung Disease grading classification. Chest. 2013; 143:694–702.7. Johannessen A, Nilsen RM, Storebó M, Gulsvik A, Eagan T, Bakke P. Comparison of 2011 and 2007 Global Initiative for Chronic Obstructive Lung Disease guidelines for predicting mortality and hospitalization. Am J Respir Crit Care Med. 2013; 188:51–59.8. Korean Academy of Tuberculosis and Respiratory Diseases. COPD guideline revised 2012. accessed on 5 February 2014. Available at http://www.lungkorea.org/thesis/guide.php.9. Agusti A, Hurd S, Jones P, Fabbri LM, Martinez F, Vogelmeier C, Vestbo J, Rodriguez-Roisin R. FAQs about the GOLD 2011 assessment proposal of COPD: a comparative analysis of four different cohorts. Eur Respir J. 2013; 42:1391–1401.10. Jung KS, Park HY, Park SY, Kim SK, Kim YK, Shim JJ, Moon HS, Lee KH, Yoo JH, Lee SD. Comparison of tiotropium plus fluticasone propionate/salmeterol with tiotropium in COPD: a randomized controlled study. Respir Med. 2012; 106:382–389.11. Calverley PM, Anderson JA, Celli B, Ferguson GT, Jenkins C, Jones PW, Yates JC, Vestbo J. TORCH Investigators. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med. 2007; 356:775–789.12. Calverley P, Pauwels R, Vestbo J, Jones P, Pride N, Gulsvik A, Anderson J, Maden C. TRial of Inhaled STeroids ANd long-acting beta2 agonists study group. Combined salmeterol and fluticasone in the treatment of chronic obstructive pulmonary disease: a randomised controlled trial. Lancet. 2003; 361:449–456.13. Yoo KH, Kim YS, Sheen SS, Park JH, Hwang YI, Kim SH, Yoon HI, Lim SC, Park JY, Park SJ, et al. Prevalence of chronic obstructive pulmonary disease in Korea: the fourth Korean National Health and Nutrition Examination Survey, 2008. Respirology. 2011; 16:659–665.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- COPD Guideline Revised 2012: Contracted Version
- Comparison of Endothelin Release in Bronchial Asthma, Chronic Obstructive Pulmonary Disease and Healthy Controls
- Management of Chronic Obstructive Pulmonary Disease (COPD)
- Chronic Obstructive Pulmonary Disease: Respiratory Review of 2014
- Bacterial etiology of acute exacerbations of chronic obstructive pulmonary disease in hospitalized patients