Cancer Res Treat.
2004 Apr;36(2):132-139.
Synthetic CDCA Derivatives-Induced Apoptosis of Stomach Cancer Cell Line SNU-1 Cells
- Affiliations
-
- 1Department of Family Medicine, Dong-A University College of Medicine, Busan, Korea. jspark@daunet.donga.ac.kr
- 2Department of Surgery, Dong-A University College of Medicine, Busan, Korea.
Abstract
- PURPOSE
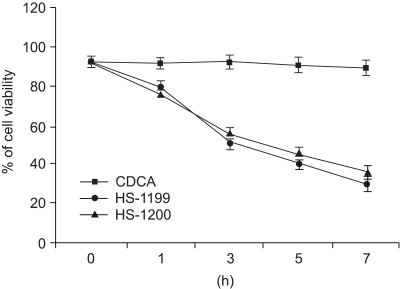
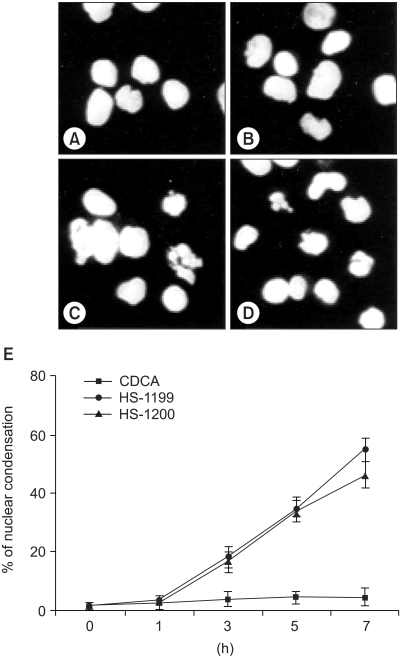
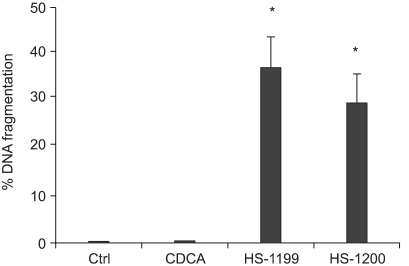
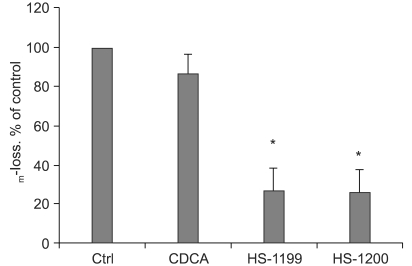
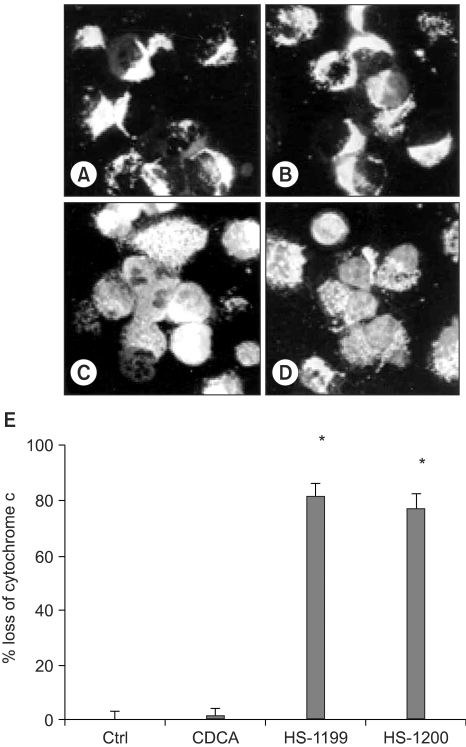
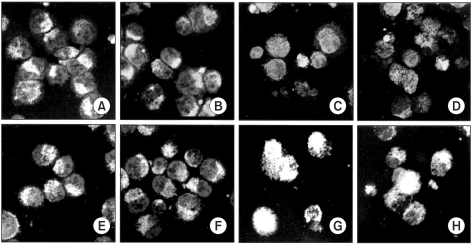
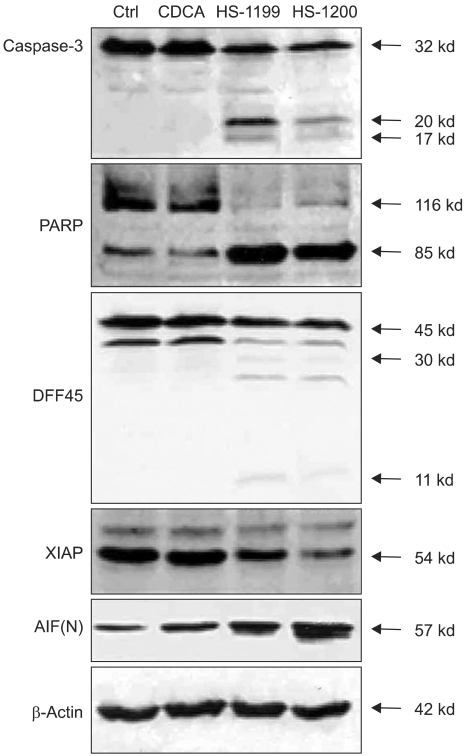
This study was conducted to explore whether CDCA derivatives induce apoptosis in a stomach cancer cell line, and to dissect the detailed mechanism underlying apoptosis. MATERIALS AND METHODS: The human stomach cancer cell line, SNU-1, cells were treated with the synthetic CDCA derivatives, HS-1199 and HS-1200. DNA and mitochondrial stains were used to detect apoptotic cells by fluorescence imaging or flow cytometry. The caspase-3 activity was measured by Western blotting. RESULTS: Both the HS-1199 and HS-1200 induced decreased viabilities of the SNU-1 cells, in time-dependent manners. The CDCA derivatives demonstrated various apoptosis hallmarks, such as mitochondrial changes reduction of MMP, cytochrome c release, and Smac/ DIABLO translocation), activation of caspase-3 (resulting in the degradation of PARP and DFF45), DNA fragmentation and nuclear condensation. CONCLUSION: The CDCA derivatives, HS-1199 and HS- 1200, both induced apoptosis of the SNU-1 gastric cancer cells in caspase- and mitochondria-dependent fashions. Many important issues relating to their therapeutic applications remain to be elucidated.
MeSH Terms
Figure
Reference
-
1. Martinez JD, Stratagoules ED, LaRue JM, Powell AA, Gause PR, Craven MT, Payne CM, Powell MB, Gerner EW, Earnest DL. Different bile acids exhibit distinct biological effects: the tumor promoter deoxycholic acid induces apoptosis and the chemopreventive agent ursodeoxycholic acid inhibits cell proliferation. Nutr Cancer. 1998; 31:111–118. PMID: 9770722.
Article2. Rust C, Karnitz LM, Paya CV, Moscat J, Simari RD, Gores GJ. The bile acid taurochenodeoxycholate activates a phosphatidylinositol 3-kinase-dependent survival signaling cascade. J Biol Chem. 2000; 275:20210–20216. PMID: 10770953.
Article3. Chu SH, Park WM, Lee KE, Pae YS. Glycochenodeoxycholic acid induces cell death in primary cultured rat hepatocyte: Apoptosis and necrosis. Korean J Physiol Pharmacol. 1999; 3:565–570.4. Powell AA, LaRue JM, Batta AK, Martinez JD. Bile acid hydrophobicity is correlated with induction of apoptosis and/or growth arrest in HCT116 cells. Biochem J. 2001; 356:481–486. PMID: 11368775.
Article5. Loogna P, Franzen L, Sipponen P, Domellof L. Effects of Helicobacter pylori and bile on N-methyl-N'-nitro-N'-nitrosoguanidine exposed antral mucosa of C57BU6 mice. Virchows Arch. 2001a; 439:661–667. PMID: 11764387.6. Loogna P, Franzen L, Sipponen P, Domellof L. Helicobacter pylori, N-methyl-N'-nitro-N'-nitrosoguanidine, and bile modulate gastric cell kinetics in experimental cancer. Virchows Arch. 2001b; 439:653–660. PMID: 11764386.
Article7. Brady LM, Beno DWA, Davis BH. Bile acid stimulation of early growth response gene and mitogen-activated protein kinase is protein kinase C-dependent. Biochem J. 1996; 316:765–769. PMID: 8670150.
Article8. LaRue JM, Stratagoules ED, Martinez JD. Deoxycholic acid-induced apoptosis is switched to necrosis by bcl-2 or calphostin C. Cancer Lett. 2000; 152:107–113. PMID: 10754212.9. Glinghammar B, Holmberg K, Rafter J. Effects of colonic luminal components on AP-1-dependent gene transcription in cultured human colon carcinoma cells. Carcinogenesis. 1999; 20:969–976. PMID: 10357775.10. Zang F, Subbaramaiah K, Altorki N, Dannenberg AJ. Dihydroxy bile acid activates the transcription of cyclooxygenase-2. J Biol Chem. 1998; 273:2424–2428. PMID: 9442092.11. Di Toro R, Campana G, Murari G, Spampinato S. Effects of specific bile acids on c-fos messenger RNA levels in human colon carcinoma Caco-2 cells. Eur J Pharm Sci. 2000; 11:291–298. PMID: 11033072.12. Choi YH, Im EO, Suh H, Jin Y, Lee WH, Yoo YH, Kim KW, Kim ND. Apoptotic activity of novel bile acid derivatives in human leukemic T cells through the activation of caspases. Int J Oncol. 2001; 18:979–984. PMID: 11295044.
Article13. Im EO, Choi YH, Paik KJ, Suh H, Jin Y, Kim KW, Yoo YH, Kim ND. Novel bile acid derivatives induce apoptosis via a p53-independent pathway in human breast carcinoma cells. Cancer Lett. 2001; 163:83–93. PMID: 11163111.
Article14. Im EO, Lee S, Suh H, Kim KW, Bae YT, Kim ND. A novel ursodeoxycholic acid derivative induces apoptosis in human MCF-7 breast cancer cells. Pharm Pharmacol Commun. 1999; 5:1–6.
Article15. Huang P, Ballal K, Plunkett W. Biochemical characterization of the protein activity responsible for high molecular weight DNA fragmentation during drug-induced apoptosis. Cancer Res. 1997; 57:3407–3414. PMID: 9270006.16. Neamati N, Fernandez A, Wright S, Kiefer J, McConkey DJ. Degradation of lamin B1 precedes oligonucleosomal DNA fragmentation in apoptotic thymocytes and isolated thymocyte nuclei. J Immunol. 1995; 154:3788–3795. PMID: 7535814.17. Konopleva M, Zhao S, Xie Z, Segall H, Younes A, Claxton DF, Estrov Z, Kornblau SM, Andreeff M. Apoptosis. Molecules and mechanisms. Adv Exp Med Biol. 1999; 457:217–236. PMID: 10500797.18. Lazebnik YA, Kaufmann SH, Desnoyers S, Poirier GG, Earnshaw WC. Cleavage of poly (ADP-ribose) polymerase by a proteinase with properties like ICE. Nature. 1994; 371:346–347. PMID: 8090205.19. Findley HW, Gu L, Yeager AM, Zhou M. Expression and regulation of Bcl-2, Bcl-xl, and Bax correlate with p53 status and sensitivity to apoptosis in childhood acute lymphoblastic leukemia. Blood. 1997; 89:2986–2993. PMID: 9108419.20. Salomons GS, Brady HJ, Verwijs-Janssen M, Van Den Berg JD, Hart AA, Behrendt H, Hahlen K, Smets LA. The Bax alpha: Bcl-2 ratio modulates the response to dexamethasone in leukaemic cells and is highly variable in childhood acute leukaemia. Int J Cancer. 1997; 71:959–965. PMID: 9185697.21. Susin SA, Lorenzo HK, Zamzami N, Marzo I, Snow BE, Brothers GM, Mangion J, Jacotot E, Costantini P, Loeffler M, Larochette N, Goodlett DR, Aebersold R, Siderovski DP, Penninger JM, Kroemer G. Molecular characterization of mitochondrial apoptosis-inducing factor. Nature. 1999; 397:441–446. PMID: 9989411.
Article22. Yerushalmi B, Dahl R, Devereaux MW, Gumpricht E, Sokol RJ. Bile acid-induced rat hepatocyte apoptosis is inhibited by antioxidants and blockers of the mitochondrial permeability transition. Hepatology. 2001; 33:616–626. PMID: 11230742.
Article23. Kroemer G, Dallaporta B, Resche-Rigon M. The mitochondrial death/life regulator in apoptosis and necrosis. Annu Rev Physiol. 1998; 60:619–642. PMID: 9558479.
Article24. Bidere N, Senik A. Caspase-independent apoptotic pathways in T lymphocytes: A minireview. Apoptosis. 2001; 6:371–375. PMID: 11483861.25. Du C, Fang M, Li L, Wang W. Smac, a mitochondrial protein that promotes cytochrome c-dependent caspase activation by eliminating IAP inhibition. Cell. 2000; 102:33–42. PMID: 10929711.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Synthetic Chenodeoxycholic Acid Derivative HS-1200-induced Apoptosis of Human Melanoma Cells
- Synthetic Bile Acid Derivative HS-1200-induced Apoptosis of Human Osteosarcoma Cells
- A Synthetic Chenodeoxycholic Acid Derivative, HS-1200-induced Apoptosis of RBL-2H3 Cells
- Modulation of Apoptosis and Cell Cycle by Synthetic Bile Acid Derivatives
- Apoptotic Effect of Co-treatment with Chios Gum Mastic and HS-1200 on G361 Human Melanoma Cell Line