Brain Tumor Res Treat.
2015 Oct;3(2):81-88. 10.14791/btrt.2015.3.2.81.
Role of Craniofacial Resection for Malignant Tumors Involving the Anterior Skull Base: Surgical Experience in a Single Institution
- Affiliations
-
- 1Department of Neurosurgery, Chonnam National University Research Institute of Medical Sciences, Chonnam National University Hwasun Hospital, Hwasun, Korea. moonks@chonnam.ac.kr
- 2Department of Otorhinolaryngology-Head and Neck Surgery, Chonnam National University Research Institute of Medical Sciences, Chonnam National University Hwasun Hospital, Hwasun, Korea.
- 3Department of Pathology, Chonnam National University Research Institute of Medical Sciences, Chonnam National University Hwasun Hospital, Hwasun, Korea.
- KMID: 2114652
- DOI: http://doi.org/10.14791/btrt.2015.3.2.81
Abstract
- BACKGROUND
Craniofacial resection (CFR) has been regarded as a standard treatment for various tumors involving the anterior skull base. The purpose of this study was to evaluate the results of CFR for the patients with anterior skull base malignancies in our hospital.
METHODS
We retrospectively analyzed 17 patients with anterior skull base malignancies treated with CFR between 2001 and 2012. Mean follow-up duration was 41 months (range, 2-103 months).
RESULTS
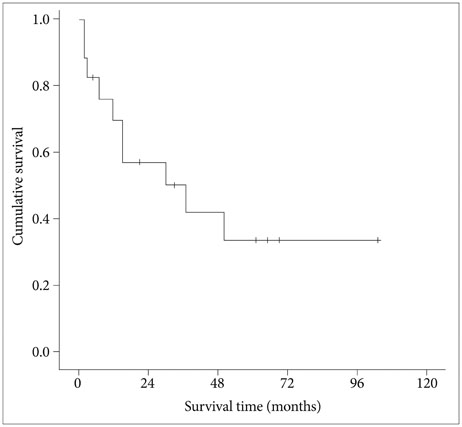
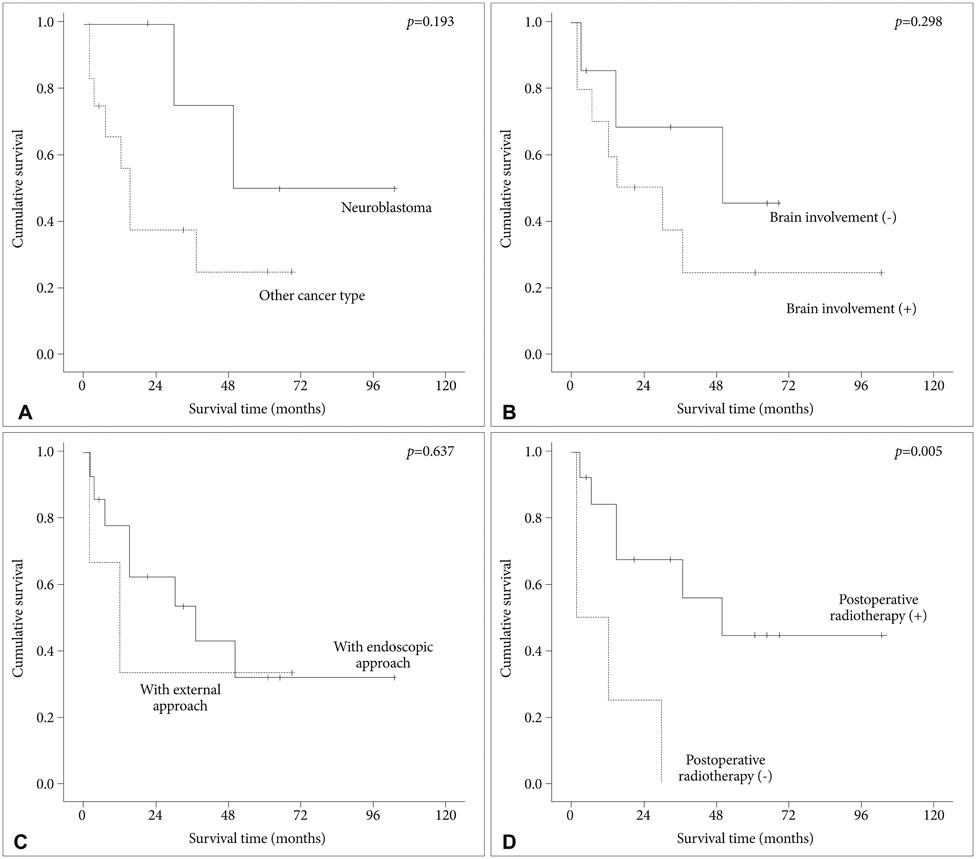
Intracranial involvement was found in 11 patients (65%) and orbital extension in 6 patients (35%). Classical bifrontal craniotomy was combined with endoscopic endonasal approach in 14 patients and external approach in 3 patients. Vascularized flap was used for reconstruction of the anterior fossa floor in 16 patients (94%). The most common pathological type was squamous cell carcinoma (6 patients). Gross total resection was achieved in all cases. Postoperative complications developed in 4 patients (24%) and included local wound problem and brain abscess. One patient with liver cirrhosis died from unexpected varix bleeding after the operation. Although postoperative treatment, such as radiotherapy or chemotherapy, was performed in 14 patients, local recurrence was seen in 6 patients. The mean overall survival time after the operation was 69.0 months (95% confidence interval: 47.5-90.5 months) with a 1-, 2-, and 5-year survival rate of 82.3%, 76.5%, and 64.7%, respectively. Postoperative radiotherapy was found to be the powerful prognostic factor for favorable survival.
CONCLUSION
Considering the higher local control rate and acceptable complication or mortality rate, CFR with adjuvant radiotherapy is a gold standard treatment option for malignant tumors involving anterior skull base, especially with extensive intracranial involvement.
Keyword
MeSH Terms
-
Brain Abscess
Carcinoma, Squamous Cell
Cranial Fossa, Anterior
Craniotomy
Drug Therapy
Follow-Up Studies
Hemorrhage
Humans
Intraoperative Complications
Liver Cirrhosis
Mortality
Orbit
Paranasal Sinus Neoplasms
Postoperative Complications
Radiotherapy
Radiotherapy, Adjuvant
Recurrence
Retrospective Studies
Skull Base*
Skull*
Survival Rate
Treatment Outcome
Varicose Veins
Wounds and Injuries
Figure
Reference
-
1. Ketcham AS, Wilkins RH, Vanburen JM, Smith RR. A combined intracranial facial approach to the paranasal sinuses. Am J Surg. 1963; 106:698–703.
Article2. Hanna E, DeMonte F, Ibrahim S, Roberts D, Levine N, Kupferman M. Endoscopic resection of sinonasal cancers with and without craniotomy: oncologic results. Arch Otolaryngol Head Neck Surg. 2009; 135:1219–1224.
Article3. Raza SM, Garzon-Muvdi T, Gallia GL, Tamargo RJ. Craniofacial resection of midline anterior skull base malignancies: a reassessment of outcomes in the modern era. World Neurosurg. 2012; 78:128–136.
Article4. Kadish S, Goodman M, Wang CC. Olfactory neuroblastoma. A clinical analysis of 17 cases. Cancer. 1976; 37:1571–1576.
Article5. Biller HF, Lawson W, Sachdev VP, Som P. Esthesioneuroblastoma: surgical treatment without radiation. Laryngoscope. 1990; 100:1199–1201.6. Harrison LB, Pfister DG, Kraus D, et al. Management of unresectable malignant tumors at the skull base using concomitant chemotherapy and radiotherapy with accelerated fractionation. Skull Base Surg. 1994; 4:127–131.
Article7. Richtsmeier WJ, Briggs RJ, Koch WM, et al. Complications and early outcome of anterior craniofacial resection. Arch Otolaryngol Head Neck Surg. 1992; 118:913–917.
Article8. Dave SP, Bared A, Casiano RR. Surgical outcomes and safety of transnasal endoscopic resection for anterior skull tumors. Otolaryngol Head Neck Surg. 2007; 136:920–927.
Article9. Eloy JA, Vivero RJ, Hoang K, et al. Comparison of transnasal endoscopic and open craniofacial resection for malignant tumors of the anterior skull base. Laryngoscope. 2009; 119:834–840.
Article10. Cantu G, Solero CL, Miceli R, et al. Anterior craniofacial resection for malignant paranasal tumors: a monoinstitutional experience of 366 cases. Head Neck. 2012; 34:78–87.
Article11. Patel SG, Singh B, Polluri A, et al. Craniofacial surgery for malignant skull base tumors: report of an international collaborative study. Cancer. 2003; 98:1179–1187.
Article12. Ganly I, Patel SG, Singh B, et al. Craniofacial resection for malignant paranasal sinus tumors: Report of an International Collaborative Study. Head Neck. 2005; 27:575–584.
Article13. Kraus DH, Shah JP, Arbit E, Galicich JH, Strong EW. Complications of craniofacial resection for tumors involving the anterior skull base. Head Neck. 1994; 16:307–312.
Article14. Mine S, Saeki N, Horiguchi K, Hanazawa T, Okamoto Y. Craniofacial Resection for Sinonasal Malignant Tumors: Statistical Analysis of Surgical Outcome over 17 Years at a Single Institution. Skull Base. 2011; 21:243–248.
Article15. Chen AM, Daly ME, El-Sayed I, et al. Patterns of failure after combined-modality approaches incorporating radiotherapy for sinonasal undifferentiated carcinoma of the head and neck. Int J Radiat Oncol Biol Phys. 2008; 70:338–343.
Article16. Bridger GP, Kwok B, Baldwin M, Williams JR, Smee RI. Craniofacial resection for paranasal sinus cancers. Head Neck. 2000; 22:772–780.
Article17. Ganly I, Patel S, Matsuo J, et al. Postoperative complications of salvage total laryngectomy. Cancer. 2005; 103:2073–2081.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Craniofacial Resection for the Anterior Skull Base Tumors
- Complications and Outcome of Craniofacial Resection for Anterior Skull Base Tumors
- Anterior and Middle Skull Base Surgery: The SNUH Experience
- Endoscopic Versus Traditional Craniofacial Resection for Patients with Sinonasal Tumors Involving the Anterior Skull Base
- Endoscopic Skull Reconstruction: Nasoseptal Flap Based Reconstructive Options