J Korean Neurosurg Soc.
2015 Oct;58(4):368-372. 10.3340/jkns.2015.58.4.368.
Chronological Changes of C-Reactive Protein Levels Following Uncomplicated, Two-Staged, Bilateral Deep Brain Stimulation
- Affiliations
-
- 1Department of Neurosurgery, St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea. sbc@catholic.ac.kr
- 2Department of Neurosurgery, Chosun University Hospital, Chosum University College of Medicine, Gwangju, Korea.
- 3The Catholic Neuroscience Institute, College of Medicine, The Catholic University of Korea, Seoul, Korea.
- KMID: 2114375
- DOI: http://doi.org/10.3340/jkns.2015.58.4.368
Abstract
OBJECTIVE
The occurrence of acute cerebral infection following deep brain stimulation (DBS) is currently being reported with elevation of C-reactive protein (CRP) level. The aim of the present study was to establish normal range of the magnitude and time-course of CRP increases following routine DBS procedures in the absence of clinical and laboratory signs of infection.
METHODS
A retrospective evaluation of serial changes of plasma CRP levels in 46 patients undergoing bilateral, two-staged DBS was performed. Because DBS was performed as a two-staged procedure involving; implantation of lead and internal pulse generator (IPG), CRP was measured preoperatively and postoperatively every 2 days until normalization of CRP (post-lead implantation day 2 and 4, post-IPG implantation day 2, 4, and 6).
RESULTS
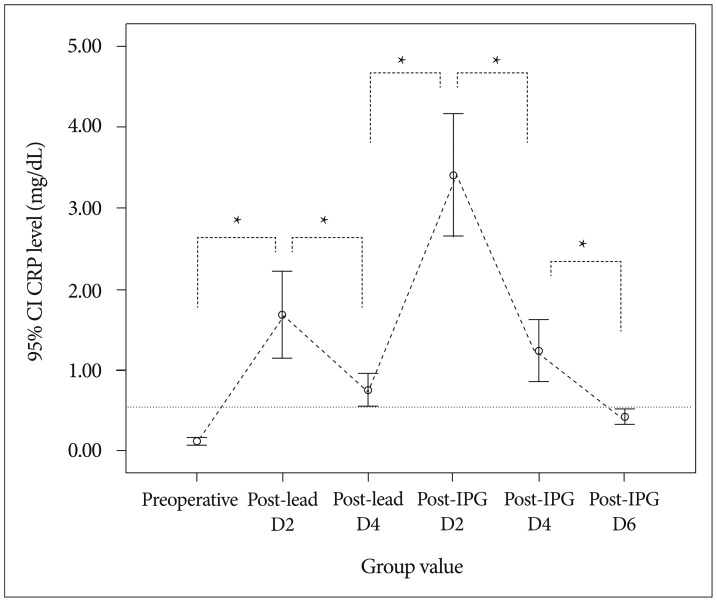
Compared with preoperative CRP levels (0.12+/-0.17 mg/dL, n=46), mean CRP levels were significantly elevated after lead insertion day 2 and 4 (1.68+/-1.83 mg/dL, n=46 and 0.76+/-0.38 mg/dL, n=16, respectively, p<0.001). The mean CRP levels at post-lead implantation day 2 were further elevated at post-IPG implantation day 2 (3.41+/-2.56 mg/dL, n=46, respectively, p<0.01). This elevation in post-IPG day 2 rapidly declined in day 4 (1.24+/-1.29 mg/dL, n=46, p<0.05) and normalized to preoperative value at day 6 (0.42+/-0.33 mg/dL, n=46, p>0.05). Mean CRP levels after IPG implantation were significantly higher in patients whose IPGs were implanted at post-lead day 3 than those at post-lead day 5-6 (3.99+/-2.80 mg/dL, n=30, and 2.31+/-1.56 mg/dL, n=16, respectively, p<0.05). However, there was no difference in post-IPG day 2 and 4 between them (p>0.05).
CONCLUSION
The mean postoperative CRP levels were highest on post-IPG insertion day 2 and decreased rapidly, returning to the normal range on post-IPG implantation day 6. The duration of post-lead implantation period influenced the magnitude of CRP elevation at post-IPG insertion day 2. Information about the normal response of CRP following DBS could help to avoid unnecessary diagnostic and therapeutic efforts.
MeSH Terms
Figure
Reference
-
1. Al-Jabi Y, El-Shawarby A. Value of C-reactive protein after neurosurgery : a prospective study. Br J Neurosurg. 2010; 24:653–659. PMID: 21070150.2. Bengzon J, Grubb A, Bune A, Hellström K, Lindström V, Brandt L. C-reactive protein levels following standard neurosurgical procedures. Acta Neurochir (Wien). 2003; 145:667–670. discussion 670-671PMID: 14520546.
Article3. Bhatia S, Oh M, Whiting T, Quigley M, Whiting D. Surgical complications of deep brain stimulation. A longitudinal single surgeon, single institution study. Stereotact Funct Neurosurg. 2008; 86:367–372. PMID: 19033705.4. Bjerknes S, Skogseid IM, Sæhle T, Dietrichs E, Toft M. Surgical site infections after deep brain stimulation surgery : frequency, characteristics and management in a 10-year period. PLoS One. 2014; 9:e105288. PMID: 25122445.5. Blomstedt P, Bjartmarz H. Intracerebral infections as a complication of deep brain stimulation. Stereotact Funct Neurosurg. 2012; 90:92–96. PMID: 22353734.
Article6. Carr WP. The role of the laboratory in rheumatology. Acute-phase proteins. Clin Rheum Dis. 1983; 9:227–239. PMID: 6191909.7. Chou YC, Lin SZ, Hsieh WA, Lin SH, Lee CC, Hsin YL, et al. Surgical and hardware complications in subthalamic nucleus deep brain stimulation. J Clin Neurosci. 2007; 14:643–649. PMID: 17532500.
Article8. Constantoyannis C, Berk C, Honey CR, Mendez I, Brownstone RM. Reducing hardware-related complications of deep brain stimulation. Can J Neurol Sci. 2005; 32:194–200. PMID: 16018154.
Article9. Deligny C, Drapier S, Verin M, Lajat Y, Raoul S, Damier P. Bilateral subthalamotomy through dbs electrodes : a rescue option for device-related infection. Neurology. 2009; 73:1243–1244. PMID: 19822876.
Article10. Du Clos TW, Mold C. The role of C-reactive protein in the resolution of bacterial infection. Curr Opin Infect Dis. 2001; 14:289–293. PMID: 11964845.
Article11. Falowski S, Ooi YC, Smith A, Verhargen Metman L, Bakay RA. An evaluation of hardware and surgical complications with deep brain stimulation based on diagnosis and lead location. Stereotact Funct Neurosurg. 2012; 90:173–180. PMID: 22678355.
Article12. Kindmark CO. Quantitative measurement of C-reactive protein in serum. Clin Chim Acta. 1969; 26:95–98. PMID: 5356609.
Article13. Kratz A, Lee-Lewandrowski E, Lewandowski K. The plasma proteins. In : Lewandrowski K, editor. Clinical Chemistry : Laboratory Management and Clinical Correlations. Philadelphia: Lippincott, Williams and Wilkins;2002. p. 531–560.14. Merello M, Cammarota A, Leiguarda R, Pikielny R. Delayed intracerebral electrode infection after bilateral STN implantation for Parkinson's disease. Case report. Mov Disord. 2001; 16:168–170. PMID: 11215583.
Article15. Mirzayan MJ, Gharabaghi A, Samii M, Tatagiba M, Krauss JK, Rosahl SK. Response of C-reactive protein after craniotomy for microsurgery of intracranial tumors. Neurosurgery. 2007; 60:621–625. discussion 625PMID: 17415198.
Article16. Mustard RA Jr, Bohnen JM, Haseeb S, Kasina R. C-reactive protein levels predict postoperative septic complications. Arch Surg. 1987; 122:69–73. PMID: 3800652.
Article17. Nathan BR, Scheld WM. The potential roles of C-reactive protein and procalcitonin concentrations in the serum and cerebrospinal fluid in the diagnosis of bacterial meningitis. Curr Clin Top Infect Dis. 2002; 22:155–165. PMID: 12520652.18. Orriss DE. Serial serum C-reactive protein levels as an indicator of infection in cardiac transplant patients. Med Lab Sci. 1988; 45:116–120. PMID: 3062304.19. Pepper J, Zrinzo L, Mirza B, Foltynie T, Limousin P, Hariz M. The risk of hardware infection in deep brain stimulation surgery is greater at impulse generator replacement than at the primary procedure. Stereotact Funct Neurosurg. 2013; 91:56–65. PMID: 23207787.
Article20. Rosahl SK, Gharabaghi A, Zink PM, Samii M. Monitoring of blood parameters following anterior cervical fusion. J Neurosurg. 2000; 92(2 Suppl):169–174. PMID: 10763687.
Article21. Schuhmann MU, Ostrowski KR, Draper EJ, Chu JW, Ham SD, Sood S, et al. The value of C-reactive protein in the management of shunt infections. J Neurosurg. 2005; 103(3 Suppl):223–230. PMID: 16238075.
Article22. Sillay KA, Larson PS, Starr PA. Deep brain stimulator hardware-related infections : incidence and management in a large series. Neurosurgery. 2008; 62:360–366. discussion 366-367PMID: 18382313.23. Son BC, Han SH, Choi YS, Kim HS, Kim MC, Yang SH, et al. Transaxillary subpectoral implantation of implantable pulse generator for deep brain stimulation. Neuromodulation. 2012; 15:260–266. discussion 266PMID: 22300254.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Deep Brain Stimulation for the Treatment of Movement Disorders
- C-reactive protein course after classical complication free total knee arthroplasty using navigation
- Erythrocyte sedimentation rate and C-reactive protein values in patients with hip arthroplasty
- Globus Pallidus Interna Deep Brain Stimulation for Chorea-Acanthocytosis
- Perioperative Changes in C-Reactive Protein Levels after Unilateral and Simultaneous Bilateral Total Knee Replacement