J Korean Soc Transplant.
2009 Sep;23(2):135-140. 10.4285/jkstn.2009.23.2.135.
Decision Factors on Mycophenolic Acid Dose after Renal Transplantation
- Affiliations
-
- 1Yonsei University Health System, Korea. ysms91@yuhs.ac
- 2Asan Medical Center, Korea.
- 3Seoul St. Mary's Hospital, Korea.
- 4Kang Dong Sacred Heart Hospital, Korea.
- 5Kunkook University Hosptial, Korea.
- 6Kyung Hee University Medical Center, Korea.
- 7Kosin University Gospel Hospital, Korea.
- 8Kyung Hee University East-West Neo Medical Center, Korea.
- 9Soon Chun Hyang University Hospital, Korea.
- 10Ajou University Hospital, Korea.
- 11Yeungnam University Medical Center, Korea.
- 12Inje University Ilsan-Paik Hospital, Korea.
- 13Chonnam National University Hospital, Korea.
- 14Chungnam National University Hospital, Korea.
- KMID: 2097080
- DOI: http://doi.org/10.4285/jkstn.2009.23.2.135
Abstract
- BACKGROUND
Triple immunosuppressant therapy including anti-metabolites is the representative immunosuppressive therapy after renal transplantation. This study is to evaluate the factors that influence Mycophenolate sodium (MPS, Myfortic, Novartis, Basel, Switzerland) dosage patterns in renal transplantation patients who take MPS as an inosine monophosphate dehydrogenase (IMPDH) among antimetabolites.
METHODS
From May 2007 to April 2008, 16 clinical departments of 14 transplantation centers in Korea retrospectively performed a survey on 650 renal transplantation recipients taking MPS. This survey collected personal information, clinical factors related to transplantation and immunosuppressive therapy.
RESULTS
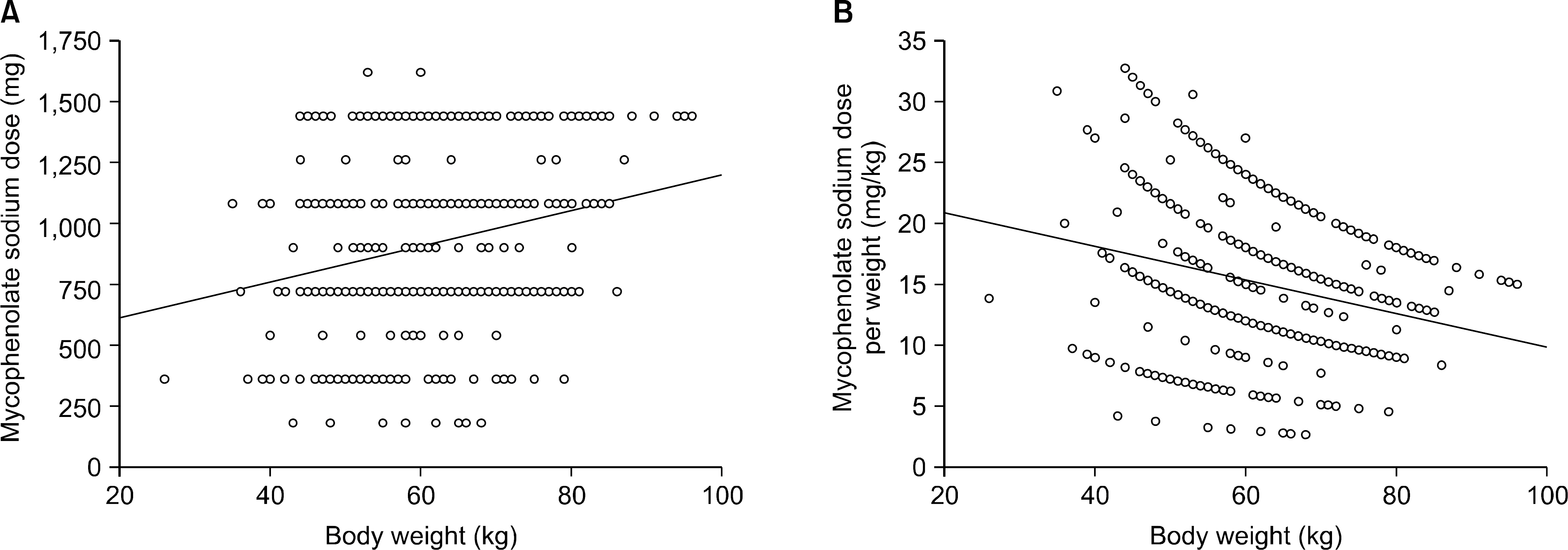
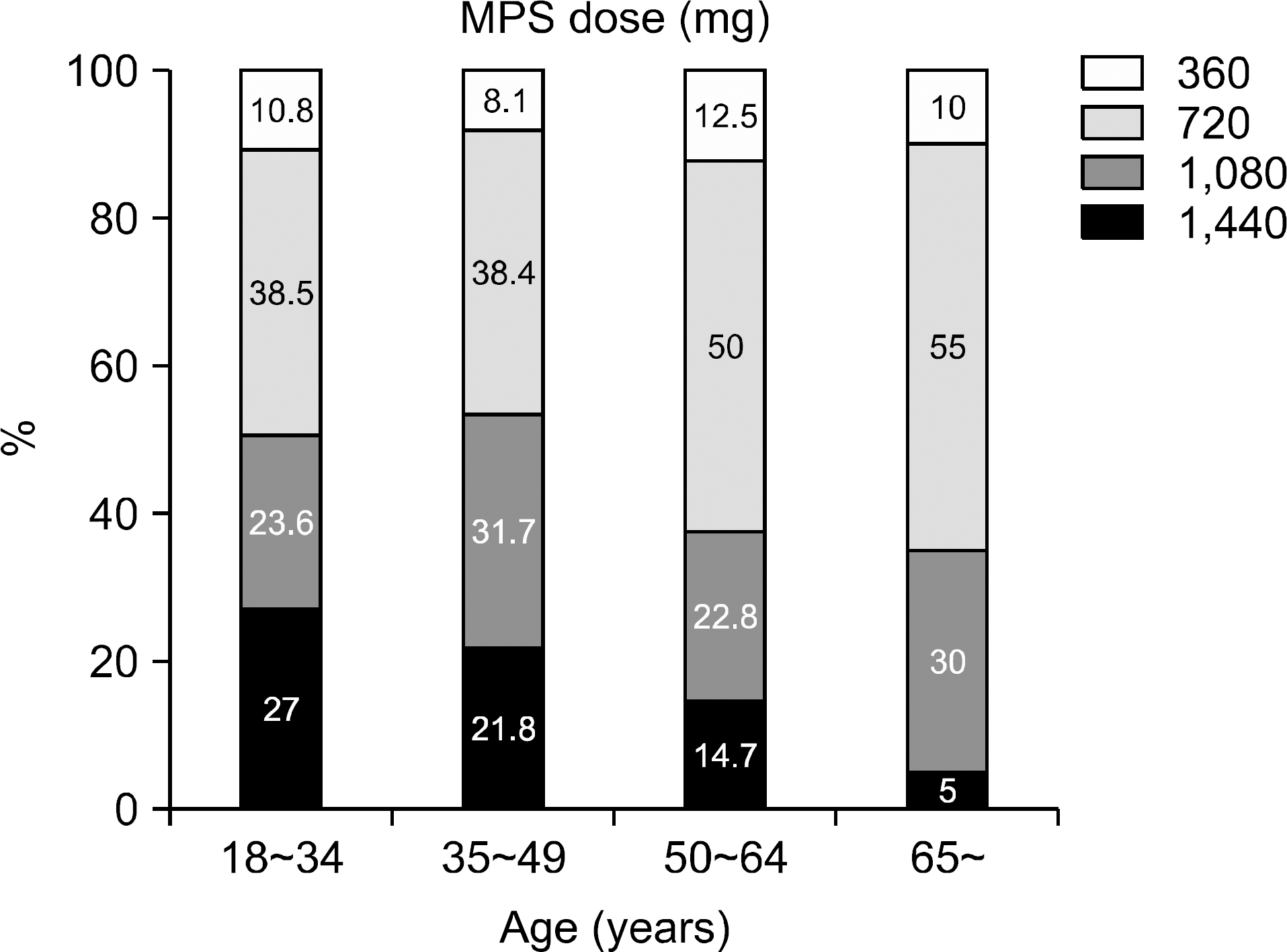
The mean age of the patients was 43.0+/-12.0 (7~75) and the study included 364 males (56.0%) and 286 females (44.0%). The average follow up period after renal transplantation was 49.5+/-53.4 (1~307) months. There were 366 (56.3%) living related cases, 145 (22.3%) living non-related cases and 139 (21.4%) deceased donor cases. Cyclosporine was the most common calcineurin inhibitor (CNI) used in combination therapy with MPS (476 cases, 73.2%) followed by tacrolimus (169 cases, 26.0%). The mean daily dose of MPS was 909.7+/-336.3 (180~1,620)mg and the mean daily dose per kg was 15.3+/-5.9 (2.65~32.73)mg/kg. The daily dose showed significant positive correlation with patient body weight but the daily dose per kg showed negative correlation. The daily dose of MPS was significantly higher in the combination therapy with cyclosporine than that with tacrolimus. The daily dose and the dose per kg decreased with increment of recipient age and post-transplant period.
CONCLUSIONS
Our study concluded that MPS dosages correlated with the combined type of CNI, post-transplant period and age.
Keyword
MeSH Terms
-
Body Weight
Calcineurin
Cyclosporine
Female
Follow-Up Studies
Humans
Inosine Monophosphate
Kidney Transplantation
Korea
Male
Mycophenolic Acid
Oxidoreductases
Retrospective Studies
Sodium
Tacrolimus
Tissue Donors
Transplants
Calcineurin
Cyclosporine
Inosine Monophosphate
Mycophenolic Acid
Oxidoreductases
Sodium
Tacrolimus
Figure
Reference
-
1). Sollinger HW. Mycophenolate mofetil for the prevention of acute rejection in primary cadaveric renal allograft recipients. U.S. Renal Transplant Mycophenolate Mofetil Study Group. Transplantation. 1995; 60:225–32.2). Ekberg H, Tedesco-Silva H, Demirbas A, Vítko S, Nashan B, Gürkan A, et al. Reduced exposure to calcineurin inhibitors in renal transplantation. N Engl J Med. 2007; 357:2562–75.
Article3). Pelletier RP, Akin B, Henry ML, Bumgardner GL, Elkhammas EA, Rajab A, et al. The impact of mycophenolate mofetil dosing patterns on clinical outcome after renal transplantation. Clin Transplant. 2003; 17:200–5.
Article4). Arns W, Breuer S, Choudhury S, Taccard G, Lee J, Binder V, et al. Enteric-coated mycophenolate sodium delivers bio-equivalent MPA exposure compared with mycophenolate mofetil. Clin Transplant. 2005; 19:199–206.
Article5). Sollinger H. Enteric-coated mycophenolate sodium: the-rapeutic equivalence to mycophenolate mofetil in de novo renal transplant patients. Transplant Proc. 2004; 36(Suppl 2S):517S–20S.
Article6). Offermann G. Five-year results of renal transplantation on immunosuppressive triple therapy with mycophenolate mofetil. Clin Transplant. 2003; 17:43–6.
Article7). Salvadori M, Holzer H, de Mattos A, Sollinger H, Arns W, Oppenheimer F, et al. Enteric-coated mycophenolate sodium is therapeutically equivalent to mycophenolate mofetil in de novo renal transplant patients. Am J Transplant. 2004; 4:31–6.8). Nashan B, Suwelack B, Ivens K, Arns W, Lhotta K, Bourbigot B, et al. Conversion to enteric-coated myco-phenolate sodium from various doses of mycophenolate mofetil: results of a prospective international multicenter trial in maintenance renal transplant patients receiving cyclosporine. Transplant Proc. 2006; 38:2856–9.
Article9). Budde K, Curtis J, Knoll G, Chan L, Neumayer HH, Seifu Y, et al. Enteric-coated mycophenolate sodium can be safely administered in maintenance renal transplant patients: results of a 1-year study. Am J Transplant. 2004; 4(2):237–43.
Article10). Budde K, Glander P, Krämer BK, Fischer W, Hoffmann U, Bauer S, et al. Conversion from mycophenolate mofetil to enteric-coated mycophenolate sodium in maintenance renal transplant recipients receiving tacrolimus: clinical, pharmacokinetic, and pharmacodynamic out-comes. Transplantation. 2007; 83:417–24.
Article11). Shehata M, Bhandari S, Venkat-Raman G, Moore R, D’Souza R, Riad H, et al. Effect of conversion from mycophenolate mofetil to enteric-coated mycophenolate sodium on maximum tolerated dose and gastrointestinal symptoms following kidney transplantation. Transpl Int. 2009; 22:821–30.
Article12). Naito T, Shinno K, Maeda T, Kagawa Y, Hashimoto H, Otsuka A, et al. Effects of calcineurin inhibitors on pharmacokinetics of mycophenolic acid and its glucuronide metabolite during the maintenance period following renal transplantation. Biol Pharm Bull. 2006; 29:275–80.
Article13). van Gelder T, Klupp J, Barten MJ, Christians U, Morris RE. Comparison of the effects of tacrolimus and cyclosporine on the pharmacokinetics of mycophenolic acid. Ther Drug Monit. 2001; 23:119–28.
Article14). Zucker K, Rosen A, Tsaroucha A, de Faria L, Roth D, Clancio G, et al. Unexpected augmentation of mycophenolic acid pharmacokinetics in renal transplant patients receiving tacrolimus and mycophenolate mofetil in combination therapy, and analogous in vitro findings. Transpl Immunol. 1997; 5:225–32.
Article15). Kaplan B, Meier-Kriesche HU, Minnick P, Bastien MC, Sechaud R, Yeh CM, et al. Randomized calcineurin inhibitor cross over study to measure the pharmacokinetics of co-administered enteric-coated mycophenolate sodium. Clin Transplant. 2005; 19:551–8.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Monitoring of Mycophenolic Acid Trough Concentration in Kidney Transplant under Cyclosporine Is Beneficial in Reducing Acute Rejection within 1 Year
- Intra-individual variability of mycophenolic acid concentration according to renal function in liver transplant recipients receiving mycophenolate monotherapy
- Therapeutic Drug Monitoring of Mycophenolic Acid
- The Efficacy and Outcome of Reduced Dose of Tacrolimus in Renal Transplantation
- Oral Ulceration an Overlooked Complication of Mycophenolate Mofetil in a Renal Transplant Recipient