J Korean Surg Soc.
2011 Aug;81(2):115-121. 10.4174/jkss.2011.81.2.115.
Up-regulation of cyclooxygenase-2-derived prostaglandin E2 in colon cancer cells resistant to 5-fluorouracil
- Affiliations
-
- 1Research Center for Resistant Cells, Chosun University School of Medicine, Gwangju, Korea.
- 2Department of Surgery, Chosun University School of Medicine, Gwangju, Korea. kjkim@chosun.ac.kr
- 3Department of Physiology, Chosun University School of Medicine, Gwangju, Korea.
- KMID: 2096665
- DOI: http://doi.org/10.4174/jkss.2011.81.2.115
Abstract
- PURPOSE
It has been suggested that constitutive up-regulation of cyclooxygenase (COX)-2 is associated with resistance to apoptosis, increased angiogenesis, and increased tumor invasiveness in various cancers including colon cancer. There are many factors involved in the resistance to 5-fluorouracil (5-FU) in colon cancer. However, little is known about the role of COX-2 in acquired resistance to 5-FU in colon cancer.
METHODS
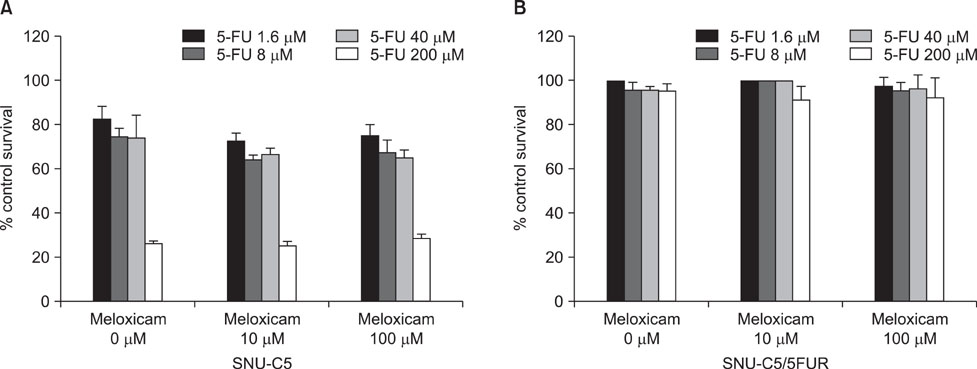
Hence we investigated whether COX-2 contribute to acquired resistance to 5-FU in colon cancer cells, using cytotoxicity assay for cell survival, reverse transcription-polymerase chain reaction (RT-PCR) for vascular endothelial growth factor (VEGF), quantitative RT-PCR for COX-1 and COX-2, and enzyme-linked immunosorbent assay for PGE2.
RESULTS
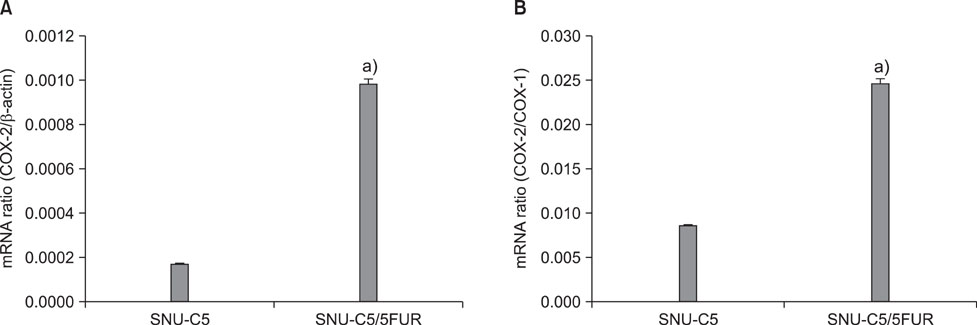
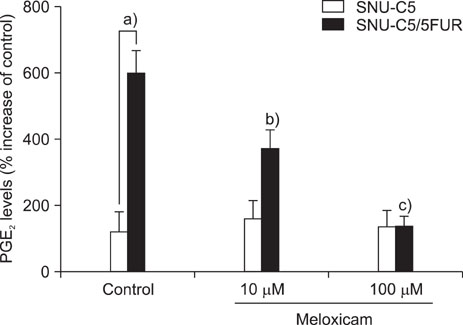
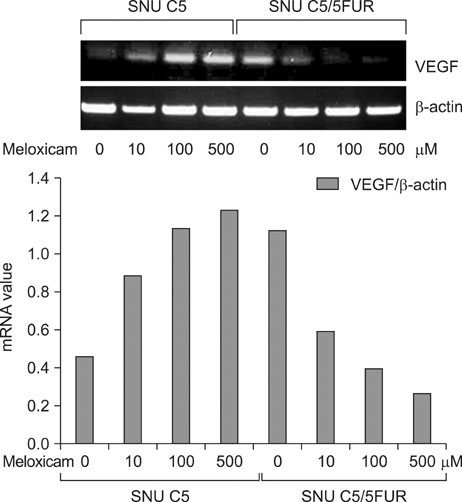
The 5-FU resistant colon cancer cells, SNU-C5/5FUR, showed increased expression of COX-2, prostaglandin E2 (PGE2), and VEGF, compared to its parental cell (SNU-C5). By treatment with meloxicam, the expression of PGE2 and VEGF was reduced significantly in the resistant cells, but not in the parent cells.
CONCLUSION
These results demonstrate that COX-2 derived PGE2 is up-regulated and COX-2 inhibitor may have an anti-angiogenic effect in the colon cancer cells resistant to 5-FU.
MeSH Terms
-
Apoptosis
Cell Survival
Colon
Colonic Neoplasms
Cyclooxygenase 2
Dinoprostone
Enzyme-Linked Immunosorbent Assay
Fluorouracil
Humans
Parents
Prostaglandin-Endoperoxide Synthases
Thiazines
Thiazoles
Up-Regulation
Vascular Endothelial Growth Factor A
Cyclooxygenase 2
Dinoprostone
Fluorouracil
Prostaglandin-Endoperoxide Synthases
Thiazines
Thiazoles
Vascular Endothelial Growth Factor A
Figure
Reference
-
1. Longley DB, Harkin DP, Johnston PG. 5-fluorouracil: mechanisms of action and clinical strategies. Nat Rev Cancer. 2003. 3:330–338.2. Popat S, Matakidou A, Houlston RS. Thymidylate synthase expression and prognosis in colorectal cancer: a systematic review and meta-analysis. J Clin Oncol. 2004. 22:529–536.3. Salonga D, Danenberg KD, Johnson M, Metzger R, Groshen S, Tsao-Wei DD, et al. Colorectal tumors responding to 5-fluorouracil have low gene expression levels of dihydropyrimidine dehydrogenase, thymidylate synthase, and thymidine phosphorylase. Clin Cancer Res. 2000. 6:1322–1327.4. Boyer J, McLean EG, Aroori S, Wilson P, McCulla A, Carey PD, et al. Characterization of p53 wild-type and null isogenic colorectal cancer cell lines resistant to 5-fluorouracil, oxaliplatin, and irinotecan. Clin Cancer Res. 2004. 10:2158–2167.5. Wang W, McLeod HL, Cassidy J. Disulfiram-mediated inhibition of NF-kappaB activity enhances cytotoxicity of 5-fluorouracil in human colorectal cancer cell lines. Int J Cancer. 2003. 104:504–511.6. Meyers M, Wagner MW, Hwang HS, Kinsella TJ, Boothman DA. Role of the hMLH1 DNA mismatch repair protein in fluoropyrimidine-mediated cell death and cell cycle responses. Cancer Res. 2001. 61:5193–5201.7. Mirjolet JF, Didelot C, Barberi-Heyob M, Merlin JL. G(1)/S but not G(0)/G(1)cell fraction is related to 5-fluorouracil cytotoxicity. Cytometry. 2002. 48:6–13.8. Brown JR, DuBois RN. COX-2: a molecular target for colorectal cancer prevention. J Clin Oncol. 2005. 23:2840–2855.9. Yamauchi T, Watanabe M, Kubota T, Hasegawa H, Ishii Y, Endo T, et al. Cyclooxygenase-2 expression as a new marker for patients with colorectal cancer. Dis Colon Rectum. 2002. 45:98–103.10. Williams CS, Mann M, DuBois RN. The role of cyclooxygenases in inflammation, cancer, and development. Oncogene. 1999. 18:7908–7916.11. Fosslien E. Biochemistry of cyclooxygenase (COX)-2 inhibitors and molecular pathology of COX-2 in neoplasia. Crit Rev Clin Lab Sci. 2000. 37:431–502.12. Subbaramaiah K, Dannenberg AJ. Cyclooxygenase 2: a molecular target for cancer prevention and treatment. Trends Pharmacol Sci. 2003. 24:96–102.13. Jacoby RF, Seibert K, Cole CE, Kelloff G, Lubet RA. The cyclooxygenase-2 inhibitor celecoxib is a potent preventive and therapeutic agent in the min mouse model of adenomatous polyposis. Cancer Res. 2000. 60:5040–5044.14. Mizutani Y, Kamoi K, Ukimura O, Kawauchi A, Miki T. Synergistic cytotoxicity and apoptosis of JTE-522, a selective cyclooxygenase-2 inhibitor, and 5-fluorouracil against bladder cancer. J Urol. 2002. 168:2650–2654.15. Wilgus TA, Breza TS Jr, Tober KL, Oberyszyn TM. Treatment with 5-fluorouracil and celecoxib displays synergistic regression of ultraviolet light B-induced skin tumors. J Invest Dermatol. 2004. 122:1488–1494.16. Jung GR, Kim KJ, Choi CH, Lee TB, Han SI, Han HK, et al. Effect of betulinic acid on anticancer drug-resistant colon cancer cells. Basic Clin Pharmacol Toxicol. 2007. 101:277–285.17. Pieters R, Huismans DR, Leyva A, Veerman AJ. Adaptation of the rapid automated tetrazolium dye based (MTT) assay for chemosensitivity testing in childhood leukemia. Cancer Lett. 1988. 41:323–332.18. Cetta F, Goetz FW. Ovarian and plasma prostaglandin E and F levels in brook trout (Salvelinus fontinalis) during pituitary-induced ovulation. Biol Reprod. 1982. 27:1216–1221.19. Arango D, Corner GA, Wadler S, Catalano PJ, Augenlicht LH. c-myc/p53 interaction determines sensitivity of human colon carcinoma cells to 5-fluorouracil in vitro and in vivo. Cancer Res. 2001. 61:4910–4915.20. Kim HJ, Lee SC, Lee IK, Kang WK, Oh ST, Chang SK. The synergistic cell killing effects by the transduction of the w-p53 Gene and 5-FU administration in colon cancer cell lines. J Korean Surg Soc. 2007. 73:481–489.21. Rand A, Glenn KS, Alvares CP, White MB, Thibodeau SM, Karnes WE Jr. p53 functional loss in a colon cancer cell line with two missense mutations (218leu and 248trp) on separate alleles. Cancer Lett. 1996. 98:183–191.22. Subbaramaiah K, Hart JC, Norton L, Dannenberg AJ. Microtubule-interfering agents stimulate the transcription of cyclooxygenase-2. Evidence for involvement of ERK1/2 AND p38 mitogen-activated protein kinase pathways. J Biol Chem. 2000. 275:14838–14845.23. Mathieu A, Remmelink M, D'Haene N, Penant S, Gaussin JF, Van Ginckel R, et al. Development of a chemoresistant orthotopichuman nonsmall cell lung carcinoma model in nude mice: analyses of tumor heterogenity in relation to the immunohistochemical levels of expression of cyclooxygenase-2, ornithine decarboxylase, lung-related resistance protein, prostaglandin E synthetase, and glutathione-S-transferase-alpha (GST)-alpha, GST-mu, and GST-pi. Cancer. 2004. 101:1908–1918.24. Mercer SJ, Di Nicolantonio F, Knight LA, Gabriel FG, Whitehouse PA, Sharma S, et al. Rapid up-regulation of cyclooxygenase-2 by 5-fluorouracil in human solid tumors. Anticancer Drugs. 2005. 16:495–500.25. Debucquoy A, Goethals L, Geboes K, Roels S, Mc Bride WH, Haustermans K. Molecular responses of rectal cancer to preoperative chemoradiation. Radiother Oncol. 2006. 80:172–177.26. Irie T, Tsujii M, Tsuji S, Yoshio T, Ishii S, Shinzaki S, et al. Synergistic antitumor effects of celecoxib with 5-fluorouracil depend on IFN-gamma. Int J Cancer. 2007. 121:878–883.27. Réti A, Pap E, Zalatnai A, Jeney A, Kralovánszky J, Budai B. Co-inhibition of cyclooxygenase-2 and dihydropyrimidine dehydrogenase by non-steroidal anti-inflammatory drugs in tumor cells and xenografts. Anticancer Res. 2009. 29:3095–3101.28. Tachimori A, Yamada N, Amano R, Ohira M, Hirakawa K. Combination therapy of S-1 with selective cyclooxygenase-2 inhibitor for liver metastasis of colorectal carcinoma. Anticancer Res. 2008. 28(2A):629–638.29. Wang D, DuBois RN. Cyclooxygenase 2-derived prostaglandin E2 regulates the angiogenic switch. Proc Natl Acad Sci U S A. 2004. 101:415–416.30. Chang SH, Liu CH, Conway R, Han DK, Nithipatikom K, Trifan OC, et al. Role of prostaglandin E2-dependent angiogenic switch in cyclooxygenase 2-induced breast cancer progression. Proc Natl Acad Sci U S A. 2004. 101:591–596.31. Ueno T, Chow LW, Toi M. Increases in circulating VEGF levels during COX-2 inhibitor treatment in breast cancer patients. Biomed Pharmacother. 2006. 60:277–279.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Upregulation of prostaglandin E2 by inducible microsomal prostaglandin E synthase-1 in colon cancer
- Expression of Cyclooxygenase - 2 in Intestinal Epithelial Cells in Response to Invasive Bacterial Infection and its Role of Epithelial Cell Apoptosis
- Prostaglandin E2 receptors as therapeutic targets in renal fibrosis
- Selective cyclooxygenase inhibitors increase paclitaxel sensitivity in taxane-resistant ovarian cancer by suppressing P-glycoprotein expression
- Adjuvant Effect of NSAIDs on the Cytotoxicity of Colon Cancer Cells to 5-FU