J Korean Surg Soc.
2009 Mar;76(3):135-143. 10.4174/jkss.2009.76.3.135.
Preventive Effect of Pentoxifylline on Cyclosporine A-Induced Collagen Synthesis in Calf Pulmonary Artery Endothelial Cells
- Affiliations
-
- 1Department of Physiology, College of Medicine, Kyung Hee University, Seoul, Korea.
- 2Department of Surgery, Ilsan Paik Hospital, Inje University College of Medicine, Goyang, Korea. ceccil@paik.ac.kr
- 3Department of Anesthesiology and Pain Medicine, East-West Neo Medical Center, College of Medicine, Kyung Hee University, Seoul, Korea.
- 4Department of Internal Medicine, Ilsan Paik Hospital, Inje University College of Medicine, Goyang, Korea.
- KMID: 2096534
- DOI: http://doi.org/10.4174/jkss.2009.76.3.135
Abstract
- PURPOSE
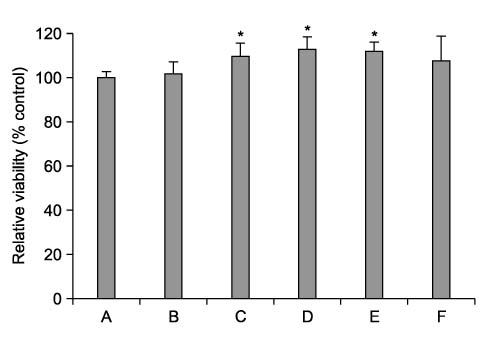
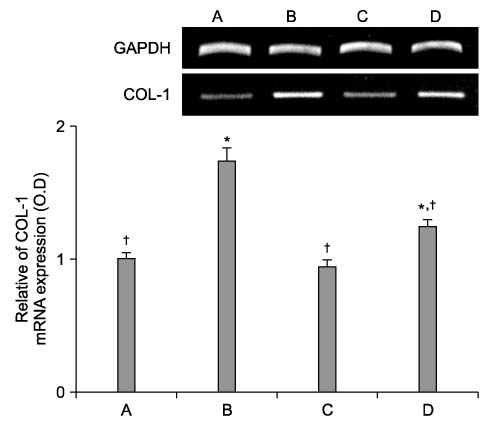
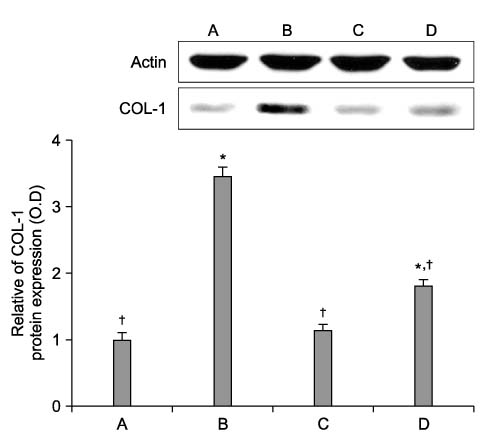
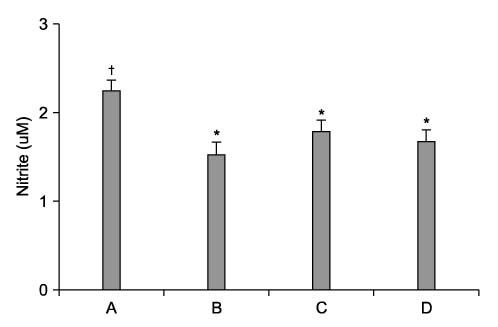
Cyclosporine A (CsA) is a potent immunosuppressive agent, and it has been used to prevent rejection of transplanted organs and to treat autoimmune diseases. Many side effects of CsA, including various types of endothelial dysfunction, have been reported. Pentoxifylline (PTX) is a non-selective phosphodiesterase inhibitor that is used for the treatment of peripheral vascular diseases. METHODS: We investigated the effect of CsA on collagen synthesis and clarified whether PTX has protective effects against CsA-induced arterial vasculopathy using calf pulmonary artery endothelial cells. This study was carried out using a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay, reverse transcription- polymerase chain reaction (RT-PCR), Western blot analysis, nitric oxide (NO) detection, and cyclic guanosine monophosphate (cGMP) enzyme immunoassay. RESULTS: CsA treatment significantly increased the expression of collagen type I mRNA and protein and decreased the production of NO and cGMP. However, pre-treatment with PTX exerted anticollagen effect by suppressing the CsA-induced formation of collagen, but this effect of PTX was not modulated by NO and cGMP. CONCLUSION: Based on the present results, it is expected that PTX may have a protective effect against CsA-induced arterial vasculopathy, although the mechanism of PTX needs to be clarified in future studies.
MeSH Terms
-
Autoimmune Diseases
Blotting, Western
Collagen
Collagen Type I
Cyclic GMP
Cyclosporine
Endothelial Cells
Guanosine Monophosphate
Immunoenzyme Techniques
Nitric Oxide
Pentoxifylline
Peripheral Vascular Diseases
Polymerase Chain Reaction
Pulmonary Artery
Rejection (Psychology)
RNA, Messenger
Tetrazolium Salts
Thiazoles
Transplants
Collagen
Collagen Type I
Cyclic GMP
Cyclosporine
Guanosine Monophosphate
Nitric Oxide
Pentoxifylline
RNA, Messenger
Tetrazolium Salts
Thiazoles
Figure
Reference
-
1. Markovic S, Raab M, Daxecker H, Griesmacher A, Karimi A, Muller MM. In vitro effects of cyclosporin A on the expression of adhesion molecules on human umbilical vein endothelial cells. Clin Chim Acta. 2002. 316:25–31.2. Oriji GK, Keiser HR. Role of nitric oxide in cyclosporine A-induced hypertension. Hypertension. 1998. 32:849–855.3. Orbe J, Fernandez L, Rodriguez JA, Rabago G, Belzunce M, Monasterio A, et al. Different expression of MMPs/TIMP-1 in human atherosclerotic lesions. Relation to plaque features and vascular bed. Atherosclerosis. 2003. 170:269–276.4. Barnes MJ, Farndale RW. Collagens and atherosclerosis. Exp Gerontol. 1999. 34:513–525.5. Beny JL, Brunet PC. Neither nitric oxide nor nitroglycerin accounts for all the characteristics of endothelially mediated vasodilatation of pig coronary arteries. Blood Vessels. 1988. 25:308–311.6. Sato K, Ozaki H, Karaki H. Changes in cytosolic calcium level in vascular smooth muscle strip measured simultaneously with contraction using fluorescent calcium indicator fura 2. J Pharmacol Exp Ther. 1988. 246:294–300.7. Mayer B, Koesling D. cGMP signalling beyond nitric oxide. Trends Pharmacol Sci. 2001. 22:546–548.8. Hohenstein B, Daniel C, Wagner A, Stasch JP, Hugo C. Stimulation of soluble guanylyl cyclase inhibits mesangial cell proliferation and matrix accumulation in experimental glomerulonephritis. Am J Physiol Renal Physiol. 2005. 288:F685–F693.9. Gossmann J, Radounikli A, Bernemann A, Schellinski O, Raab HP, Bickeboller R, et al. Pathophysiology of cyclosporine-induced nephrotoxicity in humans: a role for nitric oxide? Kidney Blood Press Res. 2001. 24:111–115.10. El-Hamamsy I, Grant M, Stevens LM, Malo O, Carrier M, Perrault LP. Cyclosporine-induced coronary endothelial dysfunction: is tetrahydrobiopterin the solution? Transplant Proc. 2005. 37:2365–2370.11. Ramzy D, Rao V, Tumiati LC, Xu N, Miriuka S, Delgado D, et al. Tetrahydrobiopterin prevents cyclosporine-induced vasomotor dysfunction. Transplantation. 2005. 79:876–881.12. Gillings DB. Pentoxifylline and intermittent claudication: review of clinical trials and cost-effectiveness analyses. J Cardiovasc Pharmacol. 1995. 25:2. S44–S50.13. Tilney NL, Milford EL, Araujo JL, Strom TB, Carpenter CB, Kirkman RL. Experience with cyclosporine and steroids in clinical renal transplantation. Ann Surg. 1984. 200:605–613.14. Wieczorek G, Bigaud M, Menninger K, Riesen S, Quesniaux V, Schuurman HJ, et al. Acute and chronic vascular rejection in nonhuman primate kidney transplantation. Am J Transplant. 2006. 6:1285–1296.15. Smith RS Jr, Agata J, Xia CF, Chao L, Chao J. Human endothelial nitric oxide synthase gene delivery protects against cardiac remodeling and reduces oxidative stress after myocardial infarction. Life Sci. 2005. 76:2457–2471.16. Dooley A, Gao B, Bradley N, Abraham DJ, Black CM, Jacobs M, et al. Abnormal nitric oxide metabolism in systemic sclerosis: increased levels of nitrated proteins and asymmetric dimethylarginine. Rheumatology (Oxford). 2006. 45:676–684.17. Dooley A, Gao B, Shi-Wen X, Abraham DJ, Black CM, Jacobs M, et al. Effect of nitric oxide and peroxynitrite on type I collagen synthesis in normal and scleroderma dermal fibroblasts. Free Radic Biol Med. 2007. 43:253–264.18. Chu AJ, Prasad JK. Up-regulation by human recombinant transforming growth factor beta-1 of collagen production in cultured dermal fibroblasts is mediated by the inhibition of nitric oxide signaling. J Am Coll Surg. 1999. 188:271–280.19. Jeanmart H, Malo O, Carrier M, Nickner C, Desjardins N, Perrault LP. Comparative study of cyclosporine and tacrolimus vs newer immunosuppressants mycophenolate mofetil and rapamycin on coronary endothelial function. J Heart Lung Transplant. 2002. 21:990–998.20. Bloom IT, Bentley FR, Spain DA, Garrison RN. An experimental study of altered nitric oxide metabolism as a mechanism of cyclosporin-induced renal vasoconstriction. Br J Surg. 1995. 82:195–198.21. Gonzalez-Santiago L, Lopez-Ongil S, Lamas S, Quereda C, Rodriguez-Puyol M, Rodriguez-Puyol D. Imbalance in endothelial vasoactive factors as a possible cause of cyclosporin toxicity: a role for endothelin-converting enzyme. J Lab Clin Med. 2000. 136:395–401.22. Kou R, Greif D, Michel T. Dephosphorylation of endothelial nitric-oxide synthase by vascular endothelial growth factor. Implications for the vascular responses to cyclosporin A. J Biol Chem. 2002. 277:29669–29673.23. Lee J, Kim SW, Kook H, Kang DG, Kim NH, Choi KC. Effects of L-arginine on cyclosporin-induced alterations of vascular NO/cGMP generation. Nephrol Dial Transplant. 1999. 14:2634–2638.24. Bernard C, Barnier P, Merval R, Esposito B, Tedgui A. Pentoxifylline selectivity inhibits tumor necrosis factor synthesis in the arterial wall. J Cardiovasc Pharmacol. 1995. 25:Suppl 2. S30–S33.25. Xiong LJ, Zhu JF, Luo DD, Zen LL, Cai SQ. Effects of pentoxifylline on the hepatic content of TGF-beta1 and collagen in Schistosomiasis japonica mice with liver fibrosis. World J Gastroenterol. 2003. 9:152–154.26. Berkenboom G, Fang ZY, Unger P, Goldman M, Fontaine J. Endothelium-dependent effects of pentoxifylline in rat aorta. Eur J Pharmacol. 1991. 193:81–86.27. Wang P, Ba ZF, Stepp KJ, Chaudry IH. Pentoxifylline attenuates the depressed endothelial cell function and vascular muscle contractility following trauma and hemorrhagic shock. J Trauma. 1995. 39:121–126.28. Zhang J, Ling Y, Tang L, Luo B, Chacko BK, Patel RP, et al. Pentoxifylline attenuation of experimental hepatopulmonary syndrome. J Appl Physiol. 2007. 102:949–955.29. Kruuse C, Jacobsen TB, Thomsen LL, Hasselbalch SG, Frandsen EK, Dige-Petersen H, et al. Effects of the non-selective phosphodiesterase inhibitor pentoxifylline on regional cerebral blood flow and large arteries in healthy subjects. Eur J Neurol. 2000. 7:629–638.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Preventive Effect of Pentoxifylline on the Oleic Acid-Induced Lung Injury
- Expression of Transforming Growth Factor-beta1 in Cyclosporine-Induced Nephropathy in Rats
- Effects of insulin and insulin-like growth factor-1 on collagen and fibronectin synthesis in endothelial cells and fibroblasts
- Effect of Pentoxifylline on Liver Fibrosis and Cell Cycle Related Proteins in Thioacetamide-Induced Rat Cirrhosis
- Anti-inflammatory Effect of Bumblebee Alcohol Extracts in CFA-Induced Rat Edema