J Periodontal Implant Sci.
2011 Aug;41(4):176-184. 10.5051/jpis.2011.41.4.176.
Dimensional change of the healed periosteum on surgically created defects
- Affiliations
-
- 1Department of Periodontology, Research Institute for Periodontal Regeneration, Yonsei University College of Dentistry, Seoul, Korea. ckkim@yuhs.ac
- KMID: 2094717
- DOI: http://doi.org/10.5051/jpis.2011.41.4.176
Abstract
- PURPOSE
The final goal of regenerative periodontal therapy is to restore the structure and function of the periodontium destroyed or lost due to periodontitis. However, the role of periosteum in periodontal regeneration was relatively neglected while bone repair in the skeleton occurs as a result of a significant contribution from the periosteum. The aim of this study is to understand the histological characteristics of periosteum and compare the native periosteum with the repaired periosteum after elevating flap or after surgical intervention with flap elevation.
METHODS
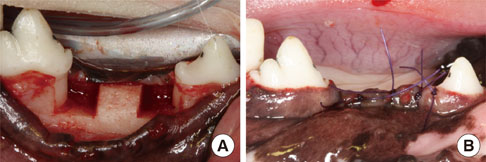
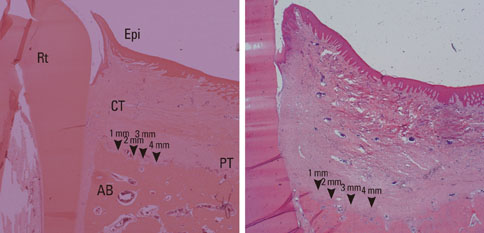
Buccal and lingual mucoperiosteal flaps were reflected to surgically create critical-size, "box-type" (4 mm width, 5 mm depth), one-wall, intrabony defects at the distal aspect of the 2nd and the mesial aspect of the 4th mandibular premolars in the right and left jaw quadrants. Animals were sacrificed after 24 weeks.
RESULTS
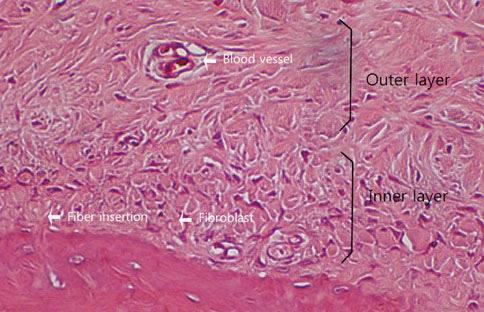
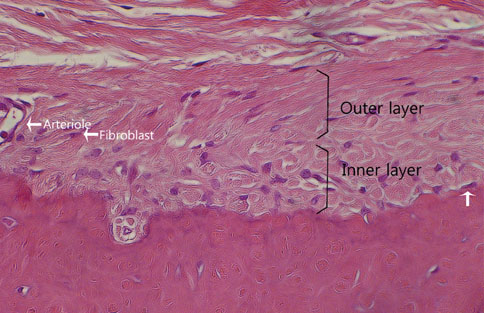
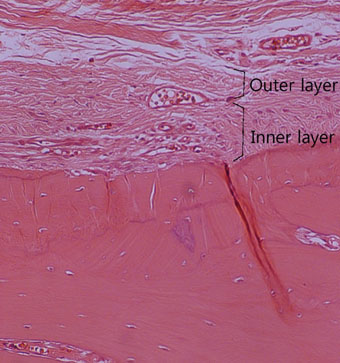
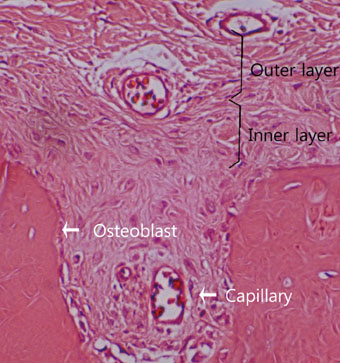
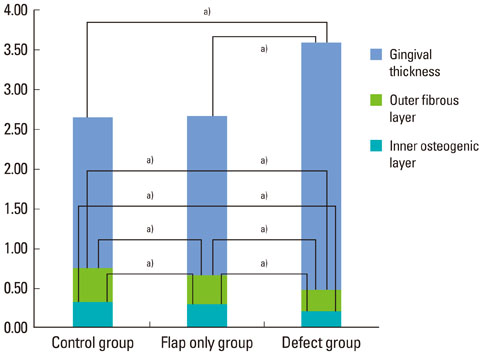
The results from this study are as follows: 1) thickness of periosteum showed difference as follows (P<0.05): control group (0.45+/-0.22 mm)>flap-elevation group (0.36+/-0.07 mm)>defect formation group (0.26+/-0.03 mm), 2) thickness of gingival tissue showed difference as follows (P<0.05): defect formation group (3.15+/-0.40 mm)>flap-elevation group (2.02+/-0.25 mm)>control group (1.88+/-0.27 mm), 3) higher cellular activity was observed in defect formation group and flap-elevation groups than control group, 4) the number of blood vessles was higher in defect formation group than control group.
CONCLUSIONS
In conclusion, prolonged operation with increased surgical trauma seems to decrease the thickness of repaired periosteum and increase the thickness of gingiva. More blood vessles and high cellular activity were observed in defect formation group.
MeSH Terms
Figure
Reference
-
1. Karring T, Nyman S, Gottlow J, Laurell L. Development of the biological concept of guided tissue regeneration--animal and human studies. Periodontol 2000. 1993. 1:26–35.
Article2. Nemcovsky CE, Artzi Z, Moses O. Rotated split palatal flap for soft tissue primary coverage over extraction sites with immediate implant placement. Description of the surgical procedure and clinical results. J Periodontol. 1999. 70:926–934.
Article3. Wang PD, Pitman DP, Jans HH. Ridge augmentation using a subepithelial connective tissue pedicle graft. Pract Periodontics Aesthet Dent. 1993. 5:47–51.4. Nemcovsky CE, Moses O, Artzi Z, Gelernter I. Clinical coverage of dehiscence defects in immediate implant procedures: three surgical modalities to achieve primary soft tissue closure. Int J Oral Maxillofac Implants. 2000. 15:843–852.5. Steiner GG, Kallet MP, Steiner DM, Roulet DN. The inverted periosteal graft. Compend Contin Educ Dent. 2007. 28:154–161.6. Allen MR, Hock JM, Burr DB. Periosteum: biology, regulation, and response to osteoporosis therapies. Bone. 2004. 35:1003–1012.
Article7. Engdahl E, Ritsilä V, Uddströmer L. Growth potential of cranial suture bone autograft. II. An experimental microscopic investigation in young rabbits. Scand J Plast Reconstr Surg. 1978. 12:125–129.
Article8. O'Driscoll SW, Salter RB. The repair of major osteochondral defects in joint surfaces by neochondrogenesis with autogenous osteoperiosteal grafts stimulated by continuous passive motion. An experimental investigation in the rabbit. Clin Orthop Relat Res. 1986. (208):131–140.9. O'Driscoll SW, Fitzsimmons JS. The importance of procedure specific training in harvesting periosteum for chondrogenesis. Clin Orthop Relat Res. 2000. (380):269–278.10. Sakaguchi Y, Sekiya I, Yagishita K, Muneta T. Comparison of human stem cells derived from various mesenchymal tissues: superiority of synovium as a cell source. Arthritis Rheum. 2005. 52:2521–2529.
Article11. De Bari C, Dell'Accio F, Vanlauwe J, Eyckmans J, Khan IM, Archer CW, et al. Mesenchymal multipotency of adult human periosteal cells demonstrated by single-cell lineage analysis. Arthritis Rheum. 2006. 54:1209–1221.
Article12. Simpson AH. The blood supply of the periosteum. J Anat. 1985. 140(Pt 4):697–704.
Article13. Squier CA, Ghoneim S, Kremenak CR. Ultrastructure of the periosteum from membrane bone. J Anat. 1990. 171:233–239.14. Campelo LD, Camara JR. Flapless implant surgery: a 10-year clinical retrospective analysis. Int J Oral Maxillofac Implants. 2002. 17:271–276.15. Ramfjord SF, Costich ER. Healing after exposure of periosteum on the alveolar process. J Periodontol. 1968. 39:199–207.
Article16. Wood DL, Hoag PM, Donnenfeld OW, Rosenfeld LD. Alveolar crest reduction following full and partial thickness flaps. J Periodontol. 1972. 43:141–144.
Article17. Wilderman MN, Pennel BM, King K, Barron JM. Histogenesis of repair following osseous surgery. J Periodontol. 1970. 41:551–565.
Article18. Van der Zee E, Oosterveld P, Van Waas MA. Effect of GBR and fixture installation on gingiva and bone levels at adjacent teeth. Clin Oral Implants Res. 2004. 15:62–65.
Article19. Casap N, Tarazi E, Wexler A, Sonnenfeld U, Lustmann J. Intraoperative computerized navigation for flapless implant surgery and immediate loading in the edentulous mandible. Int J Oral Maxillofac Implants. 2005. 20:92–98.
Article20. Hahn J. Single-stage, immediate loading, and flapless surgery. J Oral Implantol. 2000. 26:193–198.
Article21. Breitbart AS, Grande DA, Kessler R, Ryaby JT, Fitzsimmons RJ, Grant RT. Tissue engineered bone repair of calvarial defects using cultured periosteal cells. Plast Reconstr Surg. 1998. 101:567–574.
Article22. Ritsilä V, Alhopuro S, Rintala A. Bone formation with free periosteum. An experimental study. Scand J Plast Reconstr Surg. 1972. 6:51–56.23. Ritsilä V, Alhopuro S, Gylling U, Rintala A. The use of free periosteum for bone formation in congenital clefts of the maxilla. A preliminary report. Scand J Plast Reconstr Surg. 1972. 6:57–60.
Article24. Goldman HM, Smukler H. Controlled surgical stimulation of periosteum. J Periodontol. 1978. 49:518–522.
Article25. Cohen J, Lacroix P. Bone and cartilage formation by periosteum; assay of experimental autogenous grafts. J Bone Joint Surg Am. 1955. 37-A:717–730.26. Yamamiya K, Okuda K, Kawase T, Hata K, Wolff LF, Yoshie H. Tissue-engineered cultured periosteum used with platelet-rich plasma and hydroxyapatite in treating human osseous defects. J Periodontol. 2008. 79:811–818.
Article27. Schantz JT, Hutmacher DW, Chim H, Ng KW, Lim TC, Teoh SH. Induction of ectopic bone formation by using human periosteal cells in combination with a novel scaffold technology. Cell Transplant. 2002. 11:125–138.
Article28. Sakata Y, Ueno T, Kagawa T, Kanou M, Fujii T, Yamachika E, et al. Osteogenic potential of cultured human periosteum-derived cells - a pilot study of human cell transplantation into a rat calvarial defect model. J Craniomaxillofac Surg. 2006. 34:461–465.
Article29. Kim CS, Choi SH, Chai JK, Cho KS, Moon IS, Wikesjö UM, et al. Periodontal repair in surgically created intrabony defects in dogs: influence of the number of bone walls on healing response. J Periodontol. 2004. 75:229–235.
Article30. Tang XM, Chai BF. Ultrastructural investigation of osteogenic cells. Chin Med J (Engl). 1986. 99:950–956.31. Tonna EA. Response of the cellular phase of the skeleton to trauma. Periodontics. 1966. 4:105–114.32. Simon TM, Van Sickle DC, Kunishima DH, Jackson DW. Cambium cell stimulation from surgical release of the periosteum. J Orthop Res. 2003. 21:470–480.
Article33. Nobuto T, Suwa F, Kono T, Taguchi Y, Takahashi T, Kanemura N, et al. Microvascular response in the periosteum following mucoperiosteal flap surgery in dogs: angiogenesis and bone resorption and formation. J Periodontol. 2005. 76:1346–1353.
Article34. Nobuto T, Yanagihara K, Teranishi Y, Minamibayashi S, Imai H, Yamaoka A. Periosteal microvasculature in the dog alveolar process. J Periodontol. 1989. 60:709–715.
Article35. Nobuto T, Imai H, Suwa F, Kono T, Suga H, Jyoshi K, et al. Microvascular response in the periodontal ligament following mucoperiosteal flap surgery. J Periodontol. 2003. 74:521–528.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Spontaneous Bone Regeneration in Surgically Induced Bone Defects in Young Rabbits
- Role of the periosteum on bone regeneration in rabbit calvarial defects
- Tendon-to-Bone Tunnel Healing in a Rabbit Model: The Effect of Periosteum Augmentation at the Tendon-to-Bone Interface
- Depiction of the Periosteum Using Ultrashort Echo Time Pulse Sequence with Three-Dimensional Cone Trajectory and Histologic Correlation in a Porcine Model
- Bone formation e ffe ct o f HA/beta-TCP composite powders in rabbit calvarial bone defects: Histologic study