J Periodontal Implant Sci.
2010 Feb;40(1):11-18. 10.5051/jpis.2010.40.1.11.
The effects of newly formed synthetic peptide on bone regeneration in rat calvarial defects
- Affiliations
-
- 1Department of Periodontology, Research Institute for Periodontal Regeneration, Yonsei University College of Dentistry, Seoul, Korea. shchoi726@yuhs.ac
- 2Implant R&D Center, Osstem Implant Co., Ltd., Busan, Korea.
- KMID: 2094710
- DOI: http://doi.org/10.5051/jpis.2010.40.1.11
Abstract
- PURPOSE
Significant interest has emerged in the design of cell scaffolds that incorporate peptide sequences that correspond to known signaling domains in extracellular matrix and bone morphogenetic protein. The purpose of this study was to evaluate the bone regenerative effects of the synthetic peptide in a critical-size rat calvarial defect model.
METHODS
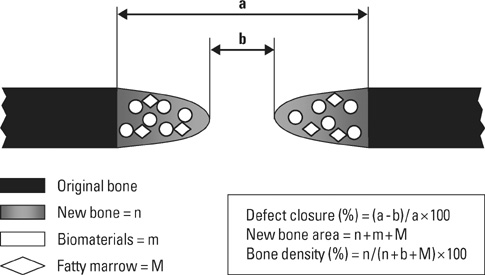
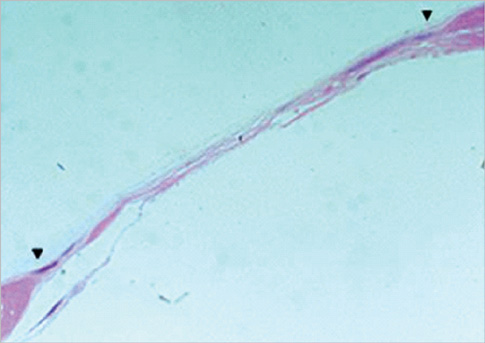
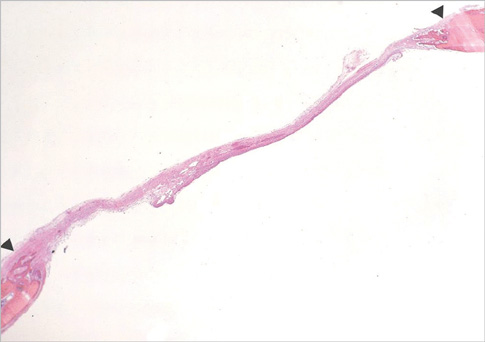
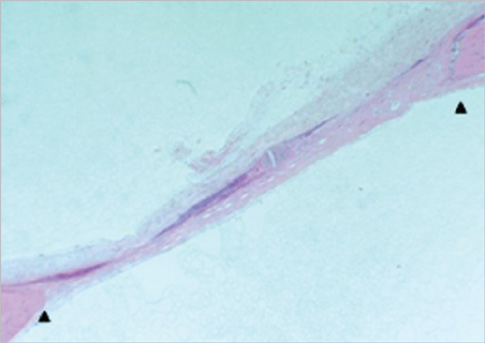
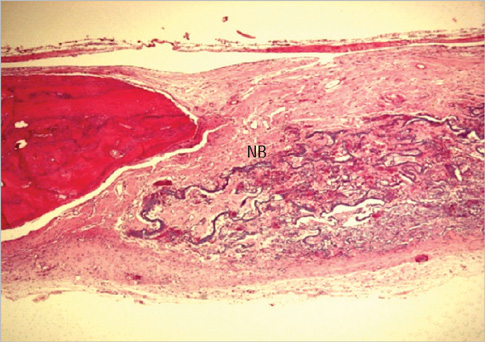
Eight millimeter diameter standardized, circular, transosseus defects created on the cranium of forty rats were implanted with synthetic peptide, collagen, or both synthetic peptide and collagen. No material was was implanted the control group. The healing of each group was evaluated histologically and histomorphometrically after 2- and 8-week healing intervals.
RESULTS
Surgical implantation of the synthetic peptide and collagen resulted in enhanced local bone formation at both 2 and 8 weeks compared to the control group. When the experimental groups were compared to each other, they showed a similar pattern of bone formation. The defect closure and new bone area were significantly different in synthetic peptide and collagen group at 8 weeks.
CONCLUSIONS
Concerning the advantages of biomaterials, synthetic peptide can be an effective biomaterial for damaged periodontal regeneration.
Keyword
MeSH Terms
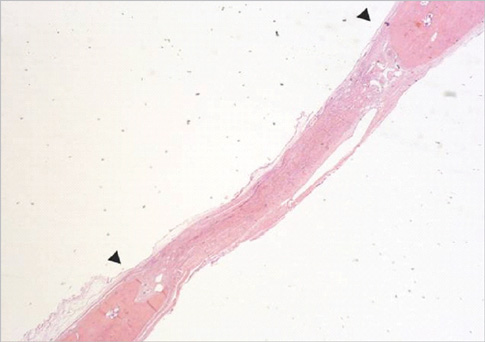
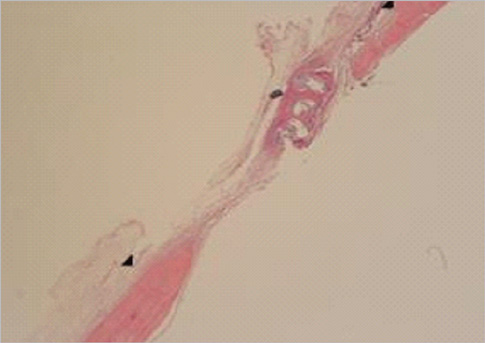
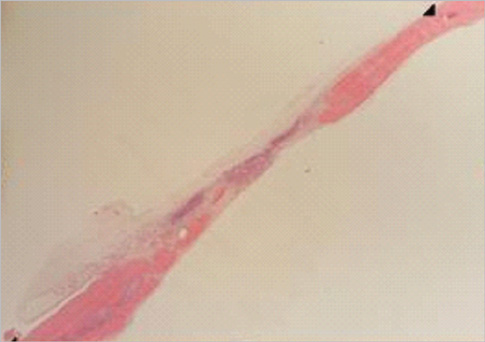
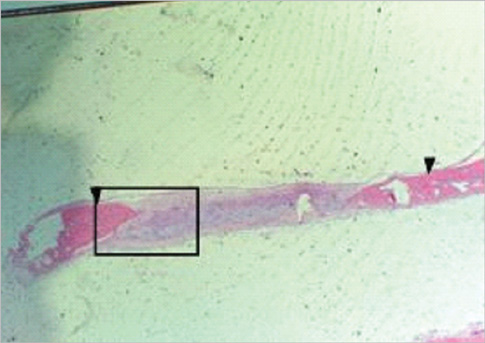
Figure
Reference
-
1. Melcher AH. On the repair potential of periodontal tissues. J Periodontol. 1976. 47:256–260.
Article2. Minabe M. A critical review of the biologic rationale for guided tissue regeneration. J Periodontol. 1991. 62:171–179.
Article3. Nyman S, Karring T, Lindhe J, Planten S. Healing following implantation of periodontitis-affected roots into gingival connective tissue. J Clin Periodontol. 1980. 7:394–401.
Article4. Urist MR. Bone: formation by autoinduction, 1965. Clin Orthop Relat Res. 2002. 395:4–10.5. Dettin M, Conconi MT, Gambaretto R, Bagno A, Di Bello C, Menti AM, et al. Effect of synthetic peptides on osteoblast adhesion. Biomaterials. 2005. 26:4507–4515.
Article6. Wozney JM, Rosen V, Celeste AJ, Mitsock LM, Whitters MJ, Kriz RW, et al. Novel regulators of bone formation: molecular clones and activities. Science. 1988. 242:1528–1534.
Article7. Celeste AJ, Iannazzi JA, Taylor RC, Hewick RM, Rosen V, Wang EA, et al. Identification of transforming growth factor beta family members present in bone-inductive protein purified from bovine bone. Proc Natl Acad Sci U S A. 1990. 87:9843–9847.
Article8. Sampath TK, Maliakal JC, Hauschka PV, Jones WK, Sasak H, Tucker RF, et al. Recombinant human osteogenic protein-1 (hOP-1) induces new bone formation in vivo with a specific activity comparable with natural bovine osteogenic protein and stimulates osteoblast proliferation and differentiation in vitro. J Biol Chem. 1992. 267:20352–20362.
Article9. Hong SJ, Kim CS, Han DK, Cho IH, Jung UW, Choi SH, et al. The effect of a fibrin-fibronectin/beta-tricalcium phosphate/recombinant human bone morphogenetic protein-2 system on bone formation in rat calvarial defects. Biomaterials. 2006. 27:3810–3816.
Article10. Saito A, Suzuki Y, Ogata S, Ohtsuki C, Tanihara M. Accelerated bone repair with the use of a synthetic BMP-2-derived peptide and bone-marrow stromal cells. J Biomed Mater Res A. 2005. 72:77–82.
Article11. Frankel AD, Pabo CO. Cellular uptake of the tat protein from human immunodeficiency virus. Cell. 1988. 55:1189–1193.
Article12. Schwarze SR, Hruska KA, Dowdy SF. Protein transduction: unrestricted delivery into all cells? Trends Cell Biol. 2000. 10:290–295.
Article13. Benoit DS, Anseth KS. The effect on osteoblast function of colocalized RGD and PHSRN epitopes on PEG surfaces. Biomaterials. 2005. 26:5209–5220.
Article14. Frame JW. A convenient animal model for testing bone substitute materials. J Oral Surg. 1980. 38:176–180.15. Caton J, Mota L, Gandini L, Laskaris B. Non-human primate models for testing the efficacy and safety of periodontal regeneration procedures. J Periodontol. 1994. 65:1143–1150.
Article16. Freeman E, Turnbull RS. The value of osseous coagulum as a graft material. J Periodontal Res. 1973. 8:229–236.
Article17. Schmitz JP, Hollinger JO. The critical size defect as an experimental model for craniomandibulofacial nonunions. Clin Orthop Relat Res. 1986. 205:299–308.
Article18. Selvig KA. Discussion: animal models in reconstructive therapy. J Periodontol. 1994. 65:1169–1172.
Article19. Saito A, Suzuki Y, Ogata S, Ohtsuki C, Tanihara M. Activation of osteo-progenitor cells by a novel synthetic peptide derived from the bone morphogenetic protein-2 knuckle epitope. Biochim Biophys Acta. 2003. 1651:60–67.
Article20. Masuko T, Iwasaki N, Yamane S, Funakoshi T, Majima T, Minami A, et al. Chitosan-RGDSGGC conjugate as a scaffold material for musculoskeletal tissue engineering. Biomaterials. 2005. 26:5339–5347.
Article21. Reddi AH. Role of morphogenetic proteins in skeletal tissue engineering and regeneration. Nat Biotechnol. 1998. 16:247–252.
Article22. Groeneveld EH, Burger EH. Bone morphogenetic proteins in human bone regeneration. Eur J Endocrinol. 2000. 142:9–21.
Article23. Wang EA, Rosen V, D'Alessandro JS, Bauduy M, Cordes P, Harada T, et al. Recombinant human bone morphogenetic protein induces bone formation. Proc Natl Acad Sci U S A. 1990. 87:2220–2224.
Article24. Hollinger JO, Schmitt JM, Buck DC, Shannon R, Joh SP, Zegzula HD, et al. Recombinant human bone morphogenetic protein-2 and collagen for bone regeneration. J Biomed Mater Res. 1998. 43:356–364.
Article25. Uludag H, Friess W, Williams D, Porter T, Timony G, D'Augusta D, et al. rhBMP-collagen sponges as osteoinductive devices: effects of in vitro sponge characteristics and protein pI on in vivo rhBMP pharmacokinetics. Ann N Y Acad Sci. 1999. 875:369–378.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Histologic Study on Healing after Implantation of several Bone Substitutes in Rat Calvarial Defects
- Histomorphometric evaluation of bone healing capacity of epigallocatechin-3-gallate-loaded β-TCP bone substitute in rabbit calvarial defects
- The Effect of Safflower Seed Extract on the Bone Formation of Calvarial Bone Model in Sprague Dawley rat
- Effects of Locally Applicated Safflower Seeds Extract on Bone Regeneration of Rat Calvarial Defects
- Effects of fibrin-binding oligopeptide on osteopromotion in rabbit calvarial defects