J Periodontal Implant Sci.
2010 Feb;40(1):3-10. 10.5051/jpis.2010.40.1.3.
Histological characteristics of newly formed cementum in surgically created one-wall intrabony defects in a canine model
- Affiliations
-
- 1Department of Periodontology, Research Institute for Periodontal Regeneration, Yonsei University College of Dentistry, Seoul, Korea. ckkim@yuhs.ac
- KMID: 2094709
- DOI: http://doi.org/10.5051/jpis.2010.40.1.3
Abstract
- PURPOSE
Periodontal regenerative therapies for defects created by severe periodontitis are mainly focused on bone regeneration. Although cementum regeneration needs to be better understood, it is believed to play an important role in periodontal regeneration. The first step toward a full understanding of cementum regeneration is to compare repaired cementum to pristine cementum. This study, which used histological techniques, was designed to focus on cementum regeneration and to compare pristine cementum to repaired cementum after surgical procedures with 8 and 24 week healing periods in a canine model.
METHODS
Buccal and lingual mucoperiosteal flaps of 10 beagle dogs were surgically reflected to create critical-sized defects. Intrabony one-wall defects, of which dimension is 4 mm width and 5 mm depth, were made at the distal aspect of mandibular second premolars and the mesial aspect of mandibular fourth premolars in the right and left jaw quadrants. Animals were sacrificed after 8 and 24 weeks post-surgery for histological specimen preparation and histometric analysis.
RESULTS
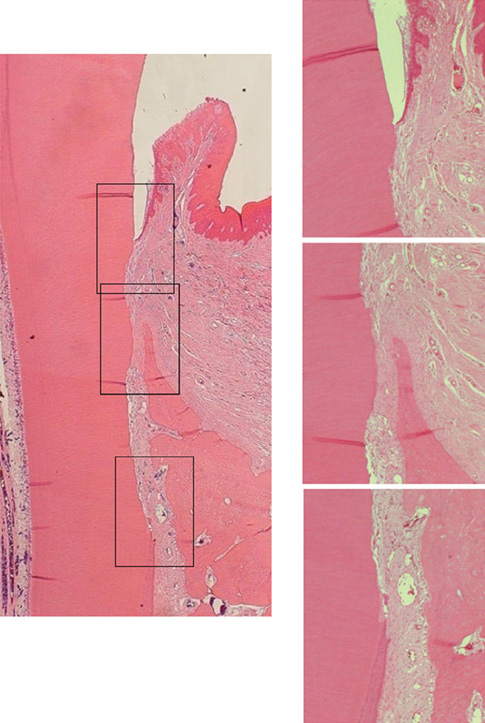
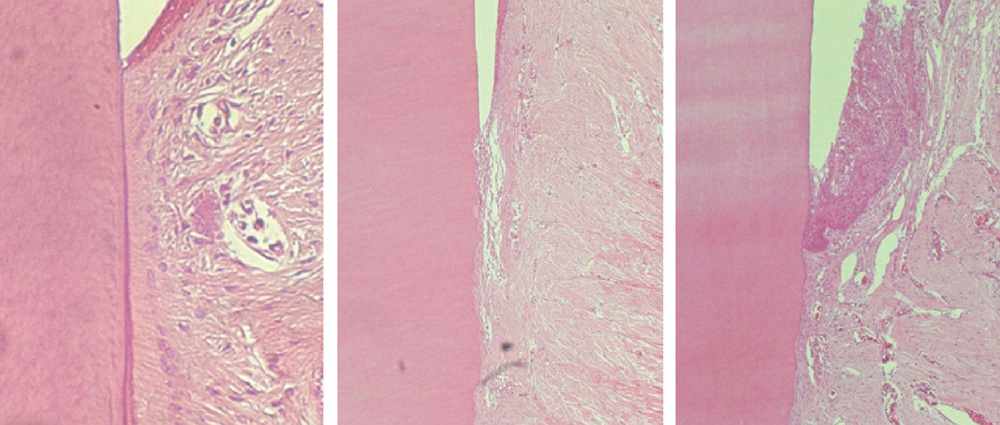
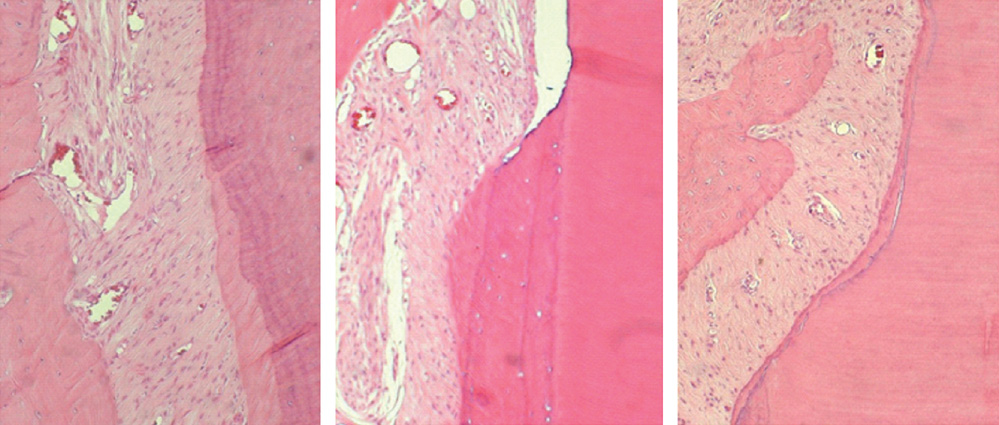
The repaired cementum was composed mostly of acellular cementum and cellular mixed fiber cementum and was thicker in the apical area than in the coronal area. The acellular cementum of the supracrestal area appeared to be amorphous. The newly formed cellular cementum was partially detached from the underlying circumpulpal dentin, which implied a weak attachment between new cementum and dentin, and this split was observed to a lesser extent in the 24 week group than in the 8 week group. The vertical height of the repaired cementum was greater in the 24 week group than in the 8 week group.
CONCLUSIONS
Within the limitations of this study, we can conclude that repaired cementum after root planing was mainly acellular cementum and cementum tissue that matured to a shape similar to pristine cementum as the healing progressed from 8 to 24 weeks.
MeSH Terms
Figure
Reference
-
1. Karring T, Nyman S, Gottlow J, Laurell L. Development of the biological concept of guided tissue regeneration: animal and human studies. Periodontol 2000. 1993. 1:26–35.
Article2. Bartold PM, McCulloch CA, Narayanan AS, Pitaru S. Tissue engineering: a new paradigm for periodontal regeneration based on molecular and cell biology. Periodontol 2000. 2000. 24:253–269.
Article3. Denton GB. The discovery of cementum. J Dent Res. 1939. 18:239. (abstract 6). In Robinson HBG. International Association for Dental Research: Proceedings of the Seventeenth General Meeting Hotel Cleveland, Cleveland, Ohio March 18 and 19, 1939. J Dent Res 1939;18:213-303.4. Bosshardt DD, Nanci A. Immunodetection of enamel- and cementum-related (bone) proteins at the enamel-free area and cervical portion of the tooth in rat molars. J Bone Miner Res. 1997. 12:367–379.
Article5. Bosshardt DD, Selvig KA. Dental cementum: the dynamic tissue covering of the root. Periodontol 2000. 1997. 13:41–75.
Article6. Bosshardt DD. Are cementoblasts a subpopulation of osteoblasts or a unique phenotype? J Dent Res. 2005. 84:390–406.
Article7. Pitaru S, McCulloch CA, Narayanan SA. Cellular origins and differentiation control mechanisms during periodontal development and wound healing. J Periodontal Res. 1994. 29:81–94.
Article8. Schroeder HE. Biological problems of regenerative cementogenesis: synthesis and attachment of collagenous matrices on growing and established root surfaces. Int Rev Cytol. 1992. 142:1–59.
Article9. Gottlow J, Karring T, Nyman S. Guided tissue regeneration following treatment of recession-type defects in the monkey. J Periodontol. 1990. 61:680–685.
Article10. Gottlow J, Nyman S, Karring T, Lindhe J. New attachment formation as the result of controlled tissue regeneration. J Clin Periodontol. 1984. 11:494–503.
Article11. Lindskog S, Blomlof L. Quality of periodontal healing. IV: Enzyme histochemical evidence for an osteoblast origin of reparative cementum. Swed Dent J. 1994. 18:181–189.12. Cochran DL, Jones A, Heijl L, Mellonig JT, Schoolfield J, King GN. Periodontal regeneration with a combination of enamel matrix proteins and autogenous bone grafting. J Periodontol. 2003. 74:1269–1281.
Article13. Donos N, Sculean A, Glavind L, Reich E, Karring T. Wound healing of degree III furcation involvements following guided tissue regeneration and/or Emdogain. A histologic study. J Clin Periodontol. 2003. 30:1061–1068.
Article14. Lindskog S, Blomlof L. Mineralized tissue-formation in periodontal wound healing. J Clin Periodontol. 1992. 19:741–748.
Article15. Araujo M, Berglundh T, Lindhe J. The periodontal tissues in healed degree III furcation defects: an experimental study in dogs. J Clin Periodontol. 1996. 23:532–541.
Article16. Araujo MG, Berglundh T, Lindhe J. On the dynamics of periodontal tissue formation in degree III furcation defects: an experimental study in dogs. J Clin Periodontol. 1997. 24:738–746.
Article17. Araujo MG, Berglundh T, Lindhe J. GTR treatment of degree III furcation defects with 2 different resorbable barriers: an experimental study in dogs. J Clin Periodontol. 1998. 25:253–259.
Article18. Schupbach P, Gaberthuel T, Lutz F, Guggenheim B. Periodontal repair or regeneration: structures of different types of new attachment. J Periodontal Res. 1993. 28:281–293.
Article19. Graziani F, Laurell L, Tonetti M, Gottlow J, Berglundh T. Periodontal wound healing following GTR therapy of dehiscence-type defects in the monkey: short-, medium- and long-term healing. J Clin Periodontol. 2005. 32:905–914.
Article20. Kim CS, Choi SH, Chai JK, Cho KS, Moon IS, Wikesjo UM, et al. Periodontal repair in surgically created intrabony defects in dogs: influence of the number of bone walls on healing response. J Periodontol. 2004. 75:229–235.
Article21. Yamamoto T, Domon T, Takahashi S, Wakita M. Cellular cementogenesis in rat molars: the role of cementoblasts in the deposition of intrinsic matrix fibers of cementum proper. Anat Embryol (Berl). 1996. 193:495–500.
Article22. Schroeder HE. Human cellular mixed stratified cementum: a tissue with alternating layers of acellular extrinsicand cellular intrinsic fiber cementum. Schweiz Monatsschr Zahnmed. 1993. 103:550–560.23. Hammarstrom L, Heijl L, Gestrelius S. Periodontal regeneration in a buccal dehiscence model in monkeys after application of enamel matrix proteins. J Clin Periodontol. 1997. 24:669–677.
Article24. Sculean A, Donos N, Brecx M, Reich E, Karring T. Treatment of intrabony defects with guided tissue regeneration and enamel-matrix-proteins: an experimental study in monkeys. J Clin Periodontol. 2000. 27:466–472.
Article25. Listgarten MA. Electron microscopic study of the junction between surgically denuded root surfaces and regenerated periodontal tissues. J Periodontal Res. 1972. 7:68–90.
Article26. Kostopoulos L, Karring T. Susceptibility of GTR-regenerated periodontal attachment to ligature-induced periodontitis. J Clin Periodontol. 2004. 31:336–340.
Article27. Bosshardt DD, Sculean A, Windisch P, Pjetursson BE, Lang NP. Effects of enamel matrix proteins on tissue formation along the roots of human teeth. J Periodontal Res. 2005. 40:158–167.
Article28. Wikesjo UM, Nilveus R. Periodontal repair in dogs. Healing patterns in large circumferential periodontal defects. J Clin Periodontol. 1991. 18:49–59.
Article29. Frank R, Fiore-Donno G, Cimasoni G, Matter J. Ultrastructural study of epithelial and connective gingival reattachment in man. J Periodontol. 1974. 45:626–635.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Effect of Calcium Sulfate on the Periodontal Healing of 2-Wall Intrabony Defects in Dogs
- Histometrical evaluation of biphasic calcium phosphate in surgically created 1-wall periodontal intrabony defects in dogs
- Periodontal repair in dogs: effect of the modified calcium sulfate paste on the 1-wall intrabony defects
- Histologic evaluation of various membranes on periodontal tissue regeneration of 1-wall intrabony defects in dogs
- The effects of composit grafts of allogenic decalcified freeze Dried bone and calcium sulfate on the healing of 1-wall intrabony defects in dogs