Korean J Physiol Pharmacol.
2012 Apr;16(2):131-138. 10.4196/kjpp.2012.16.2.131.
Cilostazol Decreases Ethanol-Mediated TNFalpha Expression in RAW264.7 Murine Macrophage and in Liver from Binge Drinking Mice
- Affiliations
-
- 1Department of Pharmacology, School of Medicine, Catholic University of Daegu, Daegu 705-718, Korea.
- 2Department of Internal Medicine, Yeungnam University College of medicine, Daegu 705-717, Korea. dreun@ynu.ac.kr
- KMID: 2071759
- DOI: http://doi.org/10.4196/kjpp.2012.16.2.131
Abstract
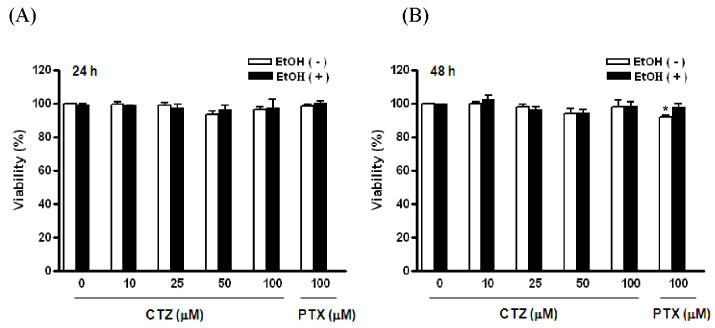
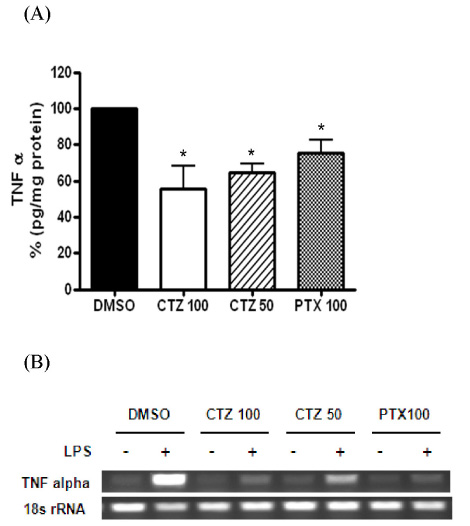
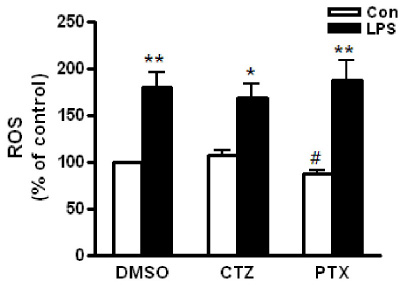
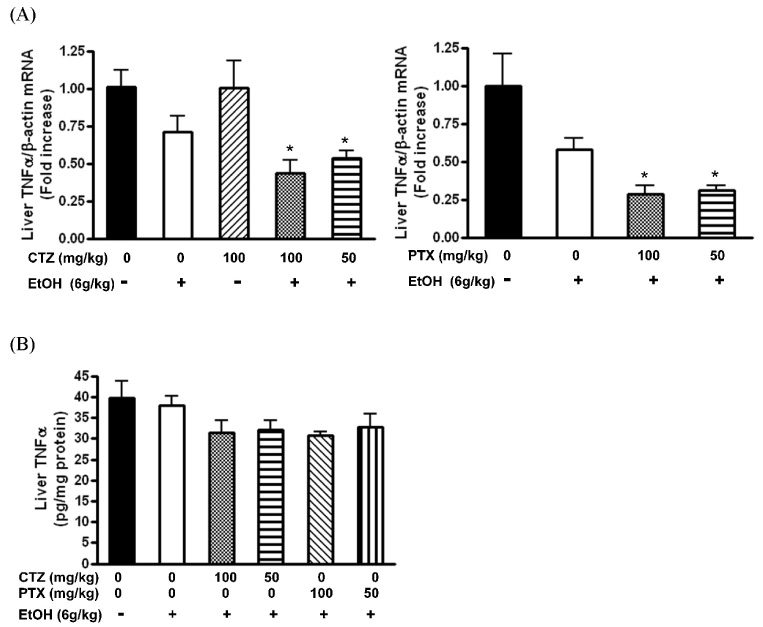
- Alcoholic hepatitis is a leading cause of liver failure in which the increased production of tumor necrosis factor alpha (TNFalpha) plays a critical role in progression of alcoholic liver disease. In the present study, we investigated the effects of cilostazol, a selective inhibitor of type III phosphodiesterase on ethanol-mediated TNFalpha production in vitro and in vivo, and the effect of cilostazol was compared with that of pentoxifylline, which is currently used in clinical trial. RAW264.7 murine macrophages were pretreated with ethanol in the presence or absence of cilostazol then, stimulated with lipopolysacchride (LPS). Cilostazol significantly suppressed the level of LPS-stimulated TNFalpha mRNA and protein with a similar degree to that by pentoxifylline. Cilostazol increased the basal AMP-activated protein kinase (AMPK) activity as well as normalized the decreased AMPK by LPS. AICAR, an AMPK activator and db-cAMP also significantly decreased TNFalpha production in RAW264.7 cells, but cilostazol did not affect the levels of intracellular cAMP and reactive oxygen species (ROS) production. The in vivo effect of cilostazol was examined using ethanol binge drinking (6 g/kg) mice model. TNFalpha mRNA and protein decreased in liver from ethanol gavaged mice compared to that from control mice. Pretreatment of mice with cilostazol or pentoxifylline further reduced the TNFalpha production in liver. These results demonstrated that cilostazol effectively decrease the ethanol-mediated TNFalpha production both in murine macrophage and in liver from binge drinking mice and AMPK may be responsible for the inhibition of TNFalpha production by cilostazol.
MeSH Terms
-
Aminoimidazole Carboxamide
AMP-Activated Protein Kinases
Animals
Binge Drinking
Ethanol
Hepatitis, Alcoholic
Liver
Liver Diseases, Alcoholic
Liver Failure
Macrophages
Mice
Pentoxifylline
Reactive Oxygen Species
Ribonucleotides
RNA, Messenger
Tetrazoles
Tumor Necrosis Factor-alpha
AMP-Activated Protein Kinases
Aminoimidazole Carboxamide
Ethanol
Pentoxifylline
RNA, Messenger
Reactive Oxygen Species
Ribonucleotides
Tetrazoles
Tumor Necrosis Factor-alpha
Figure
Reference
-
1. Thurman RG. II. Alcoholic liver injury involves activation of Kupffer cells by endotoxin. Am J Physiol. 1998. 275:G605–G611.2. Vidali M, Stewart SF, Albano E. Interplay between oxidative stress and immunity in the progression of alcohol-mediated liver injury. Trends Mol Med. 2008. 14:63–71.3. Iimuro Y, Gallucci RM, Luster MI, Kono H, Thurman RG. Antibodies to tumor necrosis factor alfa attenuate hepatic necrosis and inflammation caused by chronic exposure to ethanol in the rat. Hepatology. 1997. 26:1530–1537.4. Yin M, Wheeler MD, Kono H, Bradford BU, Gallucci RM, Luster MI, Thurman RG. Essential role of tumor necrosis factor alpha in alcohol-induced liver injury in mice. Gastroenterology. 1999. 117:942–952.5. Adachi Y, Bradford BU, Gao W, Bojes HK, Thurman RG. Inactivation of Kupffer cells prevents early alcohol-induced liver injury. Hepatology. 1994. 20:453–460.6. Tannenbaum CS, Hamilton TA. Lipopolysaccharide-induced gene expression in murine peritoneal macrophages is selectively suppressed by agents that elevate intracellular cAMP. J Immunol. 1989. 142:1274–1280.7. Thakur V, Pritchard MT, McMullen MR, Wang Q, Nagy LE. Chronic ethanol feeding increases activation of NADPH oxidase by lipopolysaccharide in rat Kupffer cells: role of increased reactive oxygen in LPS-stimulated ERK1/2 activation and TNF-alpha production. J Leukoc Biol. 2006. 79:1348–1356.8. Fischer W, Schudt C, Wendel A. Protection by phosphodiesterase inhibitors against endotoxin-induced liver injury in galactosamine-sensitized mice. Biochem Pharmacol. 1993. 45:2399–2404.9. Beavo JA, Brunton LL. Cyclic nucleotide research-still expanding after half a century. Nat Rev Mol Cell Biol. 2002. 3:710–718.10. Smith KR, Leonard D, McDonald JD, Tesfaigzi Y. Inflammation, mucous cell metaplasia, and Bcl-2 expression in response to inhaled lipopolysaccharide aerosol and effect of rolipram. Toxicol Appl Pharmacol. 2011. 253:253–260.11. Michel O, Dentener M, Cataldo D, Cantinieaux B, Vertongen F, Delvaux C, Murdoch RD. Evaluation of oral corticosteroids and phosphodiesterase-4 inhibitor on the acute inflammation induced by inhaled lipopolysaccharide in human. Pulm Pharmacol Ther. 2007. 20:676–683.12. Yoshikawa M, Suzumura A, Tamaru T, Takayanagi T, Sawada M. Effects of phosphodiesterase inhibitors on cytokine production by microglia. Mult Scler. 1999. 5:126–133.13. Akriviadis E, Botla R, Briggs W, Han S, Reynolds T, Shakil O. Pentoxifylline improves short-term survival in severe acute alcoholic hepatitis: a double-blind, placebo-controlled trial. Gastroenterology. 2000. 119:1637–1648.14. Louvet A, Diaz E, Dharancy S, Coevoet H, Texier F, Thévenot T, Deltenre P, Canva V, Plane C, Mathurin P. Early switch to pentoxifylline in patients with severe alcoholic hepatitis is inefficient in non-responders to corticosteroids. J Hepatol. 2008. 48:465–470.15. Kambayashi J, Liu Y, Sun B, Shakur Y, Yoshitake M, Czerwiec F. Cilostazol as a unique antithrombotic agent. Curr Pharm Des. 2003. 9:2289–2302.16. Liu Y, Shakur Y, Kambayashi J. Phosphodiesterases as targets for intermittent claudication. Handb Exp Pharmacol. 2011. 204:211–236.17. Lee JH, Oh GT, Park SY, Choi JH, Park JG, Kim CD, Lee WS, Rhim BY, Shin YW, Hong KW. Cilostazol reduces atherosclerosis by inhibition of superoxide and tumor necrosis factor-alpha formation in low-density lipoprotein receptor-null mice fed high cholesterol. J Pharmacol Exp Ther. 2005. 313:502–509.18. Kim MJ, Lee JH, Park SY, Hong KW, Kim CD, Kim KY, Lee WS. Protection from apoptotic cell death by cilostazol, phosphodiesterase type III inhibitor, via cAMP-dependent protein kinase activation. Pharmacol Res. 2006. 54:261–267.19. Park WS, Jung WK, Lee DY, Moon C, Yea SS, Park SG, Seo SK, Park C, Choi YH, Kim GY, Choi JS, Choi IW. Cilostazol protects mice against endotoxin shock and attenuates LPS-induced cytokine expression in RAW 264.7 macrophages via MAPK inhibition and NF-kappaB inactivation: not involved in cAMP mechanisms. Int Immunopharmacol. 2010. 10:1077–1085.20. Fujita K, Nozaki Y, Wada K, Yoneda M, Endo H, Takahashi H, Iwasaki T, Inamori M, Abe Y, Kobayashi N, Kirikoshi H, Kubota K, Saito S, Nagashima Y, Nakajima A. Effectiveness of antiplatelet drugs against experimental non-alcoholic fatty liver disease. Gut. 2008. 57:1583–1591.21. Shi L, Kishore R, McMullen MR, Nagy LE. Chronic ethanol increases lipopolysaccharide-stimulated Egr-1 expression in RAW 264.7 macrophages: contribution to enhanced tumor necrosis factor alpha production. J Biol Chem. 2002. 277:14777–14785.22. Gobejishvili L, Barve S, Joshi-Barve S, Uriarte S, Song Z, McClain C. Chronic ethanol-mediated decrease in cAMP primes macrophages to enhanced LPS-inducible NF-kappaB activity and TNF expression: relevance to alcoholic liver disease. Am J Physiol Gastrointest Liver Physiol. 2006. 291:G681–G688.23. Carson EJ, Pruett SB. Development and characterization of a binge drinking model in mice for evaluation of the immunological effects of ethanol. Alcohol Clin Exp Res. 1996. 20:132–138.24. Nakaya K, Ayaori M, Uto-Kondo H, Hisada T, Ogura M, Yakushiji E, Takiguchi S, Terao Y, Ozasa H, Sasaki M, Komatsu T, Ohsuzu F, Ikewaki K. Cilostazol enhances macrophage reverse cholesterol transport in vitro and in vivo. Atherosclerosis. 2010. 213:135–141.25. Robin MA, Demeilliers C, Sutton A, Paradis V, Maisonneuve C, Dubois S, Poirel O, Lettéron P, Pessayre D, Fromenty B. Alcohol increases tumor necrosis factor alpha and decreases nuclear factor-kappab to activate hepatic apoptosis in genetically obese mice. Hepatology. 2005. 42:1280–1290.26. Kudo H, Takahara T, Yata Y, Kawai K, Zhang W, Sugiyama T. Lipopolysaccharide triggered TNF-alpha-induced hepatocyte apoptosis in a murine non-alcoholic steatohepatitis model. J Hepatol. 2009. 51:168–175.27. Chandel NS, Trzyna WC, McClintock DS, Schumacker PT. Role of oxidants in NF-kappa B activation and TNF-alpha gene transcription induced by hypoxia and endotoxin. J Immunol. 2000. 165:1013–1021.28. Aoki C, Hattori Y, Tomizawa A, Jojima T, Kasai K. Anti-inflammatory role of cilostazol in vascular smooth muscle cells in vitro and in vivo. J Atheroscler Thromb. 2010. 17:503–509.29. Hattori Y, Suzuki K, Tomizawa A, Hirama N, Okayasu T, Hattori S, Satoh H, Akimoto K, Kasai K. Cilostazol inhibits cytokine-induced nuclear factor-kappaB activation via AMP-activated protein kinase activation in vascular endothelial cells. Cardiovasc Res. 2009. 81:133–139.30. Lambert JC, Zhou Z, Wang L, Song Z, McClain CJ, Kang YJ. Preservation of intestinal structural integrity by zinc is independent of metallothionein in alcohol-intoxicated mice. Am J Pathol. 2004. 164:1959–1966.31. Zhou Z, Wang L, Song Z, Lambert JC, McClain CJ, Kang YJ. A critical involvement of oxidative stress in acute alcohol-induced hepatic TNF-alpha production. Am J Pathol. 2003. 163:1137–1146.32. Sougioultzis S, Dalakas E, Hayes PC, Plevris JN. Alcoholic hepatitis: from pathogenesis to treatment. Curr Med Res Opin. 2005. 21:1337–1346.33. Mathurin P. Corticosteroids for alcoholic hepatitis--what's next? J Hepatol. 2005. 43:526–533.34. Hill DB, Barve S, Joshi-Barve S, McClain C. Increased monocyte nuclear factor-kappaB activation and tumor necrosis factor production in alcoholic hepatitis. J Lab Clin Med. 2000. 135:387–395.35. Yun MR, Park HM, Seo KW, Kim CE, Yoon JW, Kim CD. Cilostazol attenuates 4-hydroxynonenal-enhanced CD36 expression on murine macrophages via inhibition of NADPH oxidase-derived reactive oxygen species production. Korean J Physiol Pharmacol. 2009. 13:99–106.36. Li Y, Trush MA. Diphenyleneiodonium, an NAD(P)H oxidase inhibitor, also potently inhibits mitochondrial reactive oxygen species production. Biochem Biophys Res Commun. 1998. 253:295–299.37. Hoek JB, Pastorino JG. Ethanol, oxidative stress, and cytokine-induced liver cell injury. Alcohol. 2002. 27:63–68.38. Chacko BK, Srivastava A, Johnson MS, Benavides GA, Chang MJ, Ye Y, Jhala N, Murphy MP, Kalyanaraman B, Darley-Usmar VM. Mitochondria-targeted ubiquinone (MitoQ) decreases ethanol-dependent micro and macro hepatosteatosis. Hepatology. 2011. 54:153–163.39. Zidek Z. Adenosine - cyclic AMP pathways and cytokine expression. Eur Cytokine Netw. 1999. 10:319–328.40. Haraguchi S, Good RA, Day-Good NK. A potent immunosuppressive retroviral peptide: cytokine patterns and signaling pathways. Immunol Res. 2008. 41:46–55.41. Aroor AR, Jackson DE, Shukla SD. Elevated activation of ERK1 and ERK2 accompany enhanced liver injury following alcohol binge in chronically ethanol-fed rats. Alcohol Clin Exp Res. 2011. 35:2128–2138.42. Song Z, Deaciuc I, Song M, Lee DY, Liu Y, Ji X, McClain C. Silymarin protects against acute ethanol-induced hepatotoxicity in mice. Alcohol Clin Exp Res. 2006. 30:407–413.43. Yamashina S, Wheeler MD, Rusyn I, Ikejima K, Sato N, Thurman RG. Tolerance and sensitization to endotoxin in Kupffer cells caused by acute ethanol involve interleukin-1 receptor-associated kinase. Biochem Biophys Res Commun. 2000. 277:686–690.44. Nishiyama D, Ikejima K, Honda H, Hirose M, Takei Y, Sato N. Acute ethanol administration down-regulates toll-like receptor-4 in the murine liver. Hepatol Res. 2002. 23:130–137.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Chronic-Binge Ethanol Drinking Exacerbates Liver Damage through Oxidative/Nitrosative Stress
- Obesity and binge alcohol intake are deadly combination to induce steatohepatitis: A model of high-fat diet and binge ethanol intake
- DICAM Inhibits Activation of Macrophage by Lipopolysaccharide
- TNF-alpha and IL-12 Secretion in Macrophages in Response to Spores of Bacillus anthracis Sterne
- Construction of the Structural Equation Model on Binge Drinking among Korean Undergraduate Students