Korean J Physiol Pharmacol.
2011 Oct;15(5):291-297. 10.4196/kjpp.2011.15.5.291.
Inhibitory Actions of HERG Currents by the Immunosuppressant Drug Cyclosporin A
- Affiliations
-
- 1Department of Pharmacology, Institute for Medical Sciences, Chonbuk National University Medical School, Jeonju 561-180, Korea. bhchoi@jbnu.ac.kr
- 2Department of Physiology, Medical Research Center, College of Medicine, The Catholic University of Korea, Seoul 137-701, Korea.
- 3Department of Pharmaceutical Engineering, Gyeongnam National University of Science and Technology, Jinju 660-758, Korea.
- 4Institute of Molecular Medicine and Genetics, Department of Neurology, Medical College of Georgia, Georgia Health Sciences University, Augusta, GA 30912, USA.
- 5Department of Physiology, Institute of Bioscience and Biotechnology, Kangwon National University College of Medicine, Chuncheon 200-701, Korea.
- 6Department of Physiology, Research Institute for Biomacromolecules, University of Ulsan College of Medicine, Seoul 138-736, Korea.
- KMID: 2071732
- DOI: http://doi.org/10.4196/kjpp.2011.15.5.291
Abstract
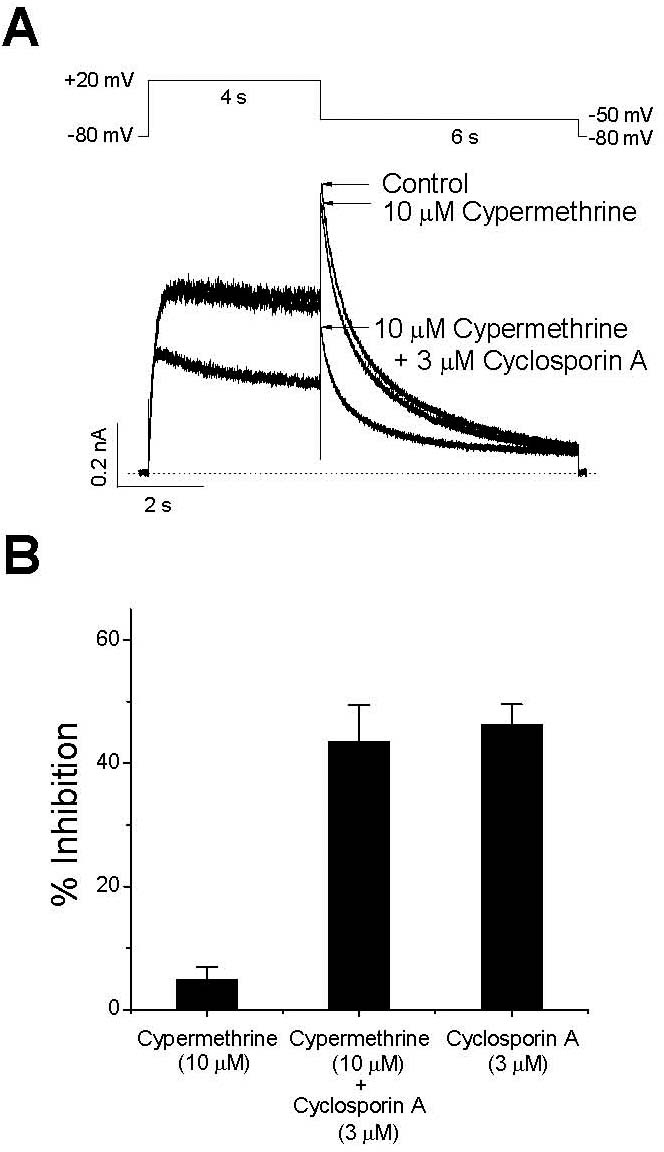
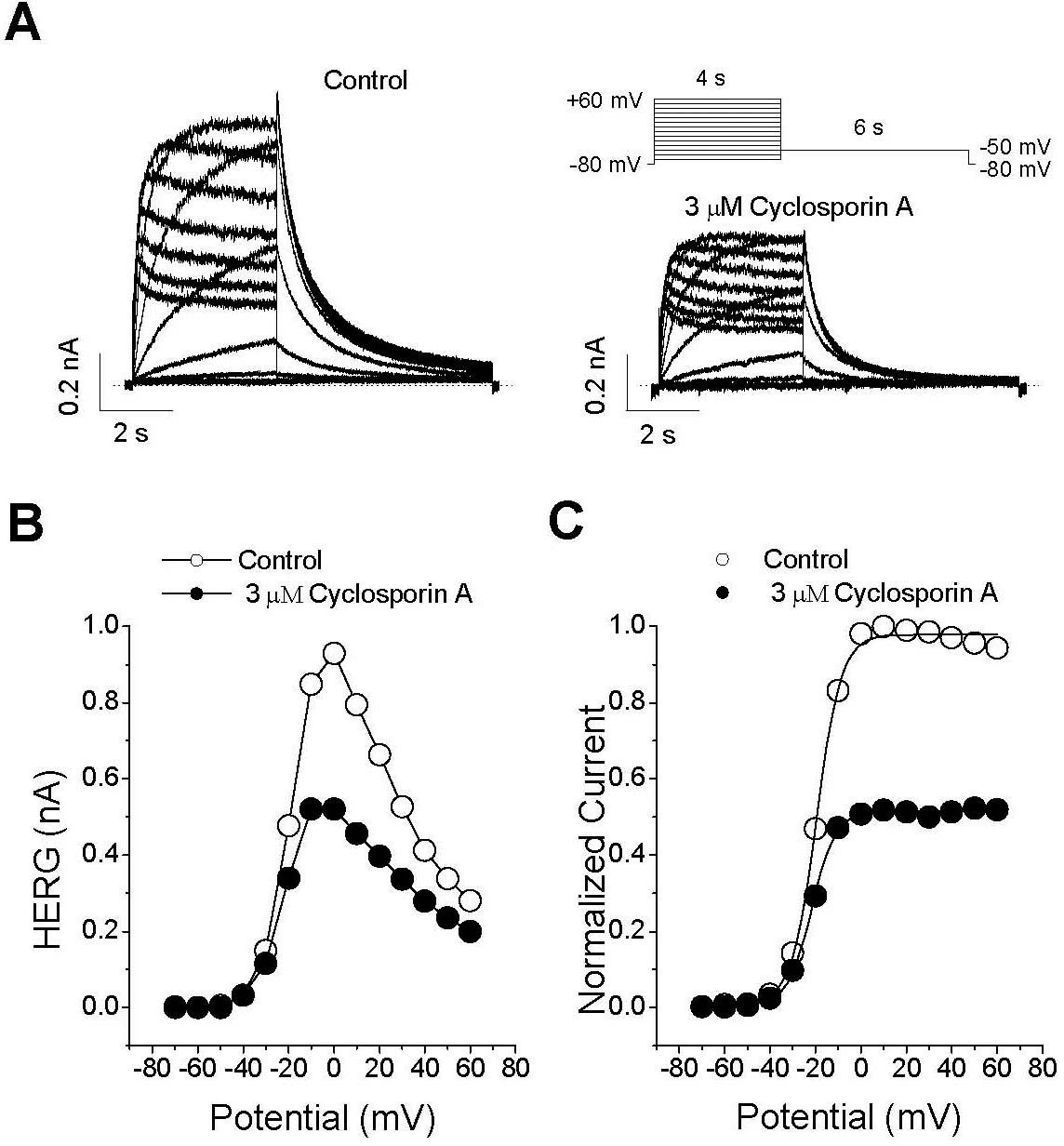
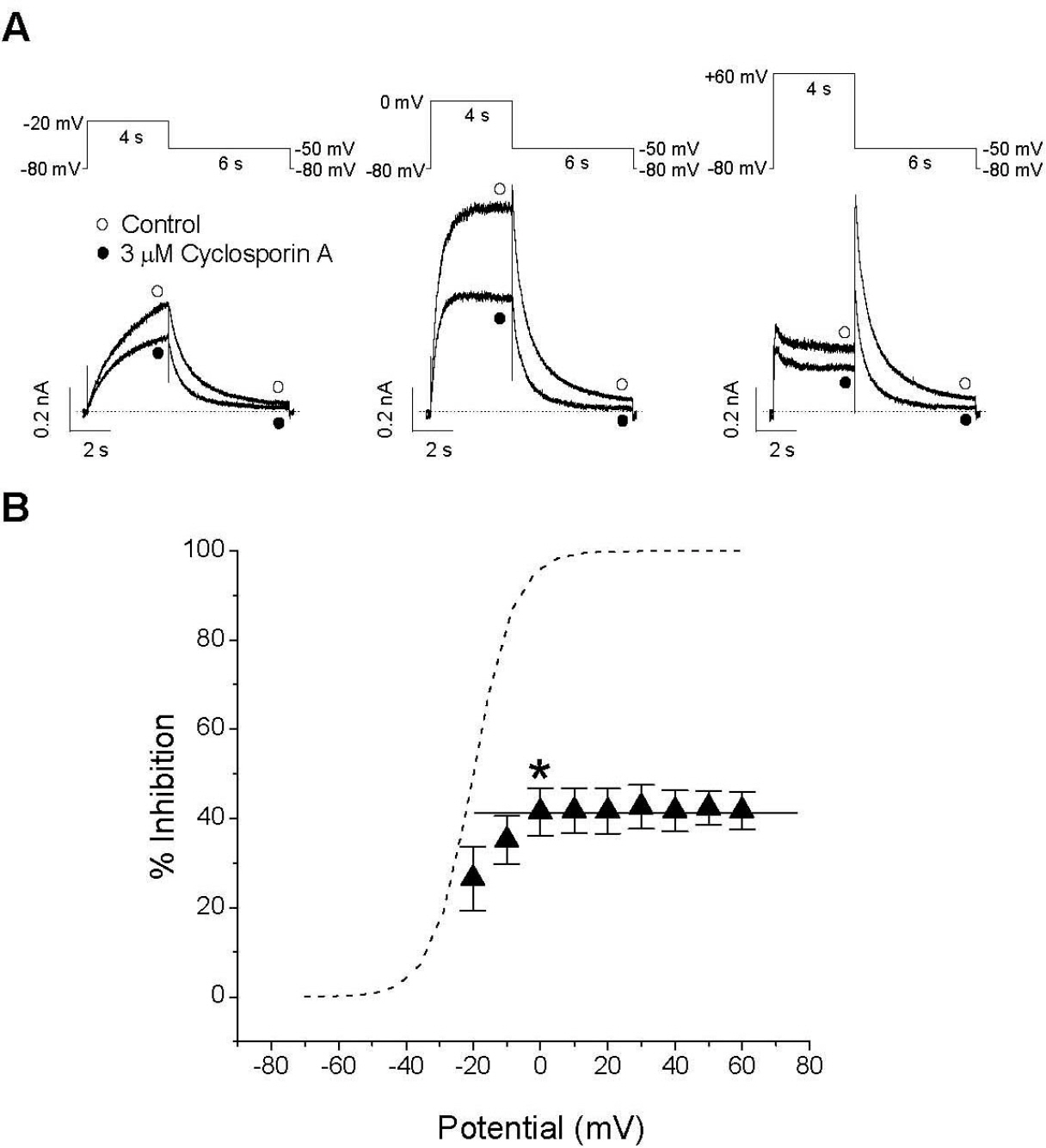
- The effect of cyclosporin A (CsA), an immunosuppressant, on human ether-a-go-go-related gene (HERG) channel as it is expressed in human embryonic kidney cells was studied using a whole-cell, patch-clamp technique. CsA inhibited the HERG channel in a concentration-dependent manner, with an IC50 value and a Hill coefficient of 3.17 microM and 0.89, respectively. Pretreatment with cypermethrine, a calcineurin inhibitor, had no effect on the CsA-induced inhibition of the HERG channel. The CsA-induced inhibition of HERG channels was voltage-dependent, with a steep increase over the voltage range of the channel opening. However, the inhibition exhibited voltage independence over the voltage range of fully activated channels. CsA blocked the HERG channels predominantly in the open and inactivated states rather than in the closed state. Results of the present study suggest that CsA acts directly on the HERG channel as an open-channel blocker, and it acts independently of its effect on calcineurin activity.
MeSH Terms
Figure
Cited by 1 articles
-
Taxifolin Glycoside Blocks Human
ether-a-go-go Related Gene K+ Channels
Jihyun Yun, Hyemi Bae, Sun Eun Choi, Jung-Ha Kim, Young Wook Choi, Inja Lim, Chung Soo Lee, Min Won Lee, Jae-Hong Ko, Seong Jun Seo, Hyoweon Bang
Korean J Physiol Pharmacol. 2013;17(1):37-42. doi: 10.4196/kjpp.2013.17.1.37.
Reference
-
References
1. Sigal NH, Dumont FJ. Cyclosporin A, FK-506, and rapamycin: pharmacologic probes of lymphocyte signal transduction. Annu Rev Immunol. 1992; 10:519–560.
Article2. Steiner JP, Dawson TM, Fotuhi M, Snyder SH. Immunophilin regulation of neurotransmitter release. Mol Med. 1996; 2:325–333.
Article3. Matsuda S, Koyasu S. Mechanisms of action of cyclosporine. Immunopharmacology. 2000; 47:119–125.
Article4. Italia JL, Bhardwaj V, Kumar MN. Disease, destination, dose and delivery aspects of ciclosporin: the state of the art. Drug Discov Today. 2006; 11:846–854.
Article5. Taub⊘ll E, Gerdts R, Gjerstad L. Cyclosporin A and brain excitability studied in vitro. Epilepsia. 1998; 39:687–691.6. Wong M, Yamada KA. Cyclosporine induces epileptiform activity in an in vitro seizure model. Epilepsia. 2000; 41:271–276.
Article7. Damjanovich S, Aszalós A, Mulhern SA, Szöllösi J, Balázs M, Trón L, Fulwyler MJ. Cyclosporin depolarizes human lymphocytes: earliest observed effect on cell metabolism. Eur J Immunol. 1987; 17:763–768.
Article8. Tordai A, Or R, Gelfand EW. Effects of changes in membrane potential on the cyclosporin-induced inhibition of T-cell proliferation. Biochem Biophys Res Commun. 1992; 185:363–369.
Article9. Panyi G, Gaspar R, Krasznai Z, ter Horst JJ, Ameloot M, Aszalos A, Steels P, Damjanovich S. Immunosuppressors inhibit voltage-gated potassium channels in human peripheral blood lymphocytes. Biochem Biophys Res Commun. 1996; 221:254–258.
Article10. Onuma H, Lu YF, Tomizawa K, Moriwaki A, Tokuda M, Hatase O, Matsui H. A calcineurin inhibitor, FK506, blocks voltagegated calcium channel-dependent LTP in the hippocampus. Neurosci Res. 1998; 30:313–319.
Article11. Sanguinetti MC, Jurkiewicz NK. Two components of cardiac delayed rectifier K+ current. Differential sensitivity to block by class III antiarrhythmic agents. J Gen Physiol. 1990; 96:195–215.12. Trudeau MC, Warmke JW, Ganetzky B, Robertson GA. HERG, a human inward rectifier in the voltage-gated potassium channel family. Science. 1995; 269:92–95.
Article13. Curran ME, Splawski I, Timothy KW, Vincent GM, Green ED, Keating MT. A molecular basis for cardiac arrhythmia: HERG mutations cause long QT syndrome. Cell. 1995; 80:795–803.
Article14. Viskin S. Long QT syndromes and torsade de pointes. Lancet. 1999; 354:1625–1633.
Article15. Suessbrich H, Waldegger S, Lang F, Busch AE. Blockade of HERG channels expressed in Xenopus oocytes by the histamine receptor antagonists terfenadine and astemizole. FEBS Lett. 1996; 385:77–80.
Article16. Taglialatela M, Castaldo P, Pannaccione A, Giorgio G, Annunziato L. Human ether-a-gogo related gene (HERG) K+ channels as pharmacological targets: present and future implications. Biochem Pharmacol. 1998; 55:1741–1746.17. Suessbrich H, Schönherr R, Heinemann SH, Attali B, Lang F, Busch AE. The inhibitory effect of the antipsychotic drug haloperidol on HERG potassium channels expressed in Xenopus oocytes. Br J Pharmacol. 1997; 120:968–974.18. Zhou Z, Gong Q, Ye B, Fan Z, Makielski JC, Robertson GA, January CT. Properties of HERG channels stably expressed in HEK 293 cells studied at physiological temperature. Biophys J. 1998; 74:230–241.
Article19. Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981; 391:85–100.
Article20. Cayabyab FS, Schlichter LC. Regulation of an ERG K+ current by Src tyrosine kinase. J Biol Chem. 2002; 277:13673–13681.21. Cockerill SL, Tobin AB, Torrecilla I, Willars GB, Standen NB, Mitcheson JS. Modulation of hERG potassium currents in HEK-293 cells by protein kinase C. Evidence for direct phosphorylation of pore forming subunits. J Physiol. 2007; 581:479–493.
Article22. Jo SH, Hong HK, Chong SH, Won KH, Jung SJ, Choe H. Clomipramine block of the hERG K+ channel: accessibility to F656 and Y652. Eur J Pharmacol. 2008; 592:19–25.23. Lee SY, Kim YJ, Kim KT, Choe H, Jo SH. Blockade of HERG human K+ channels and IKr of guinea-pig cardiomyocytes by the antipsychotic drug clozapine. Br J Pharmacol. 2006; 148:499–509.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Acepromazine inhibits hERG potassium ion channels expressed in human embryonic kidney 293 cells
- Block of hERG K+ Channel by Classic Histamine H1 Receptor Antagonist Chlorpheniramine
- Response of I(Kr) and hERG Currents to the Antipsychotics Tiapride and Sulpiride
- Inhibition of the Human Ether-a-go-go-related Gene (HERG) K+ Channels by Lindera erythrocarpa
- Taxifolin Glycoside Blocks Human ether-a-go-go Related Gene K+ Channels