Korean J Physiol Pharmacol.
2011 Oct;15(5):251-258. 10.4196/kjpp.2011.15.5.251.
Lactosylceramide Mediates the Expression of Adhesion Molecules in TNF-alpha and IFNgamma-stimulated Primary Cultured Astrocytes
- Affiliations
-
- 1Department of Pharmacology, College of Medicine, Dankook University, Cheonan 330-714, Korea.
- 2Translational Research Center, Institute of Bio-Science and Technology, Dankook University, Cheonan 330-714, Korea.
- 3Institute of Natural Medicine, Hallym University, Chuncheon 200-702, Korea. hwsuh@hallym.ac.kr
- 4Department of Pharmacology, College of Medicine, Hallym University, Chuncheon 200-702, Korea.
- KMID: 2071726
- DOI: http://doi.org/10.4196/kjpp.2011.15.5.251
Abstract
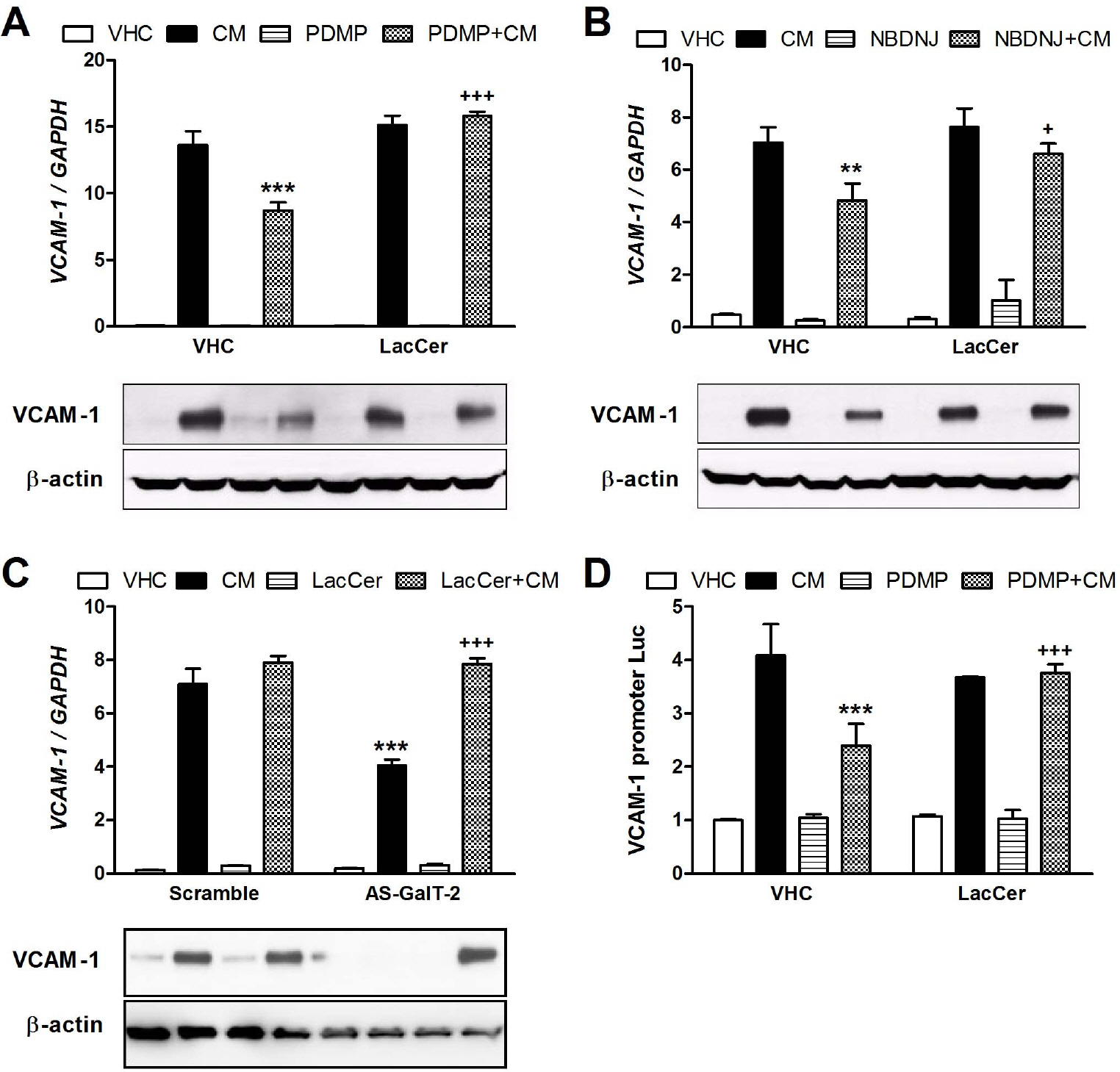
- Here we have investigated how lactosylceramide (LacCer) modulates gene expression of adhesion molecules in TNF-alpha and IFNgamma (CM)-stimulated astrocytes. We have observed that stimulation of astrocytes with CM increased the gene expression of ICAM-1 and VCAM-1. D-Threo-1-phenyl- 2-decanoylamino-3-morpholino-1-propanol (PDMP) and N-butyldeoxynojirimycin (NBDNJ), inhibitors of glucosylceramide synthase (GLS) and LacCer synthase (galactosyltransferase, GalT-2), inhibited the gene expression of ICAM-1 and VCAM-1 and activation of their gene promoter induced by CM, which were reversed by exogenously supplied LacCer. Silencing of GalT-2 gene using its antisense oligonucleotides also attenuated CM-induced ICAM-1 and VCAM-1 expression, which were reversed by LacCer. PDMP treatment and silencing of GalT-2 gene significantly reduced CM-induced luciferase activities in NF-KB, AP-1, GAS, and STAT-3 luciferase vectors-transfected cells. In addition, LacCer reversed the inhibition of NF-KB and STAT-1 luciferase activities by PDMP. Taken together, our results suggest that LacCer may play a crucial role in the expression of ICAM-1 and VCAM-1 via modulating transcription factors, such as NF-KB, AP-1, STAT-1, and STAT-3 in CM-stimulated astrocytes.
Keyword
MeSH Terms
-
1-Deoxynojirimycin
Antigens, CD
Astrocytes
Galactosyltransferases
Gene Expression
Glucosyltransferases
Intercellular Adhesion Molecule-1
Lactosylceramides
Luciferases
Morpholines
NF-kappa B
Oligonucleotides, Antisense
Transcription Factor AP-1
Transcription Factors
Tumor Necrosis Factor-alpha
Vascular Cell Adhesion Molecule-1
1-Deoxynojirimycin
Antigens, CD
Galactosyltransferases
Glucosyltransferases
Intercellular Adhesion Molecule-1
Lactosylceramides
Luciferases
Morpholines
NF-kappa B
Oligonucleotides, Antisense
Transcription Factor AP-1
Transcription Factors
Tumor Necrosis Factor-alpha
Vascular Cell Adhesion Molecule-1
Figure
Reference
-
References
1. Lee SJ, Benveniste EN. Adhesion molecule expression and regulation on cells of the central nervous system. J Neuroimmunol. 1999; 98:77–88.
Article2. Rosenman SJ, Shrikant P, Dubb L, Benveniste EN, Ransohoff RM. Cytokine-induced expression of vascular cell adhesion molecule-1 (VCAM-1) by astrocytes and astrocytoma cell lines. J Immunol. 1995; 154:1888–1899.3. Won JS, Singh AK, Singh I. Lactosylceramide: a lipid second messenger in neuroinflammatory disease. J Neurochem. 2007; 103 Suppl. 1:180–191.
Article4. Giri S, Jatana M, Rattan R, Won JS, Singh I, Singh AK. Galactosylsphingosine (psychosine)-induced expression of cytokine-mediated inducible nitric oxide synthases via AP-1 and C/EBP: implications for Krabbe disease. FASEB J. 2002; 16:661–672.5. Pannu R, Singh AK, Singh I. A novel role of lactosylceramide in the regulation of tumor necrosis factor alpha-mediated proliferation of rat primary astrocytes. Implications for astrogliosis following neurotrauma. J Biol Chem. 2005; 280:13742–13751.6. Pannu R, Won JS, Khan M, Singh AK, Singh I. A novel role of lactosylceramide in the regulation of lipopolysaccharide/interferon-gamma-mediated inducible nitric oxide synthase gene expression: implications for neuroinflammatory diseases. J Neurosci. 2004; 24:5942–5954.7. Won JS, Im YB, Khan M, Singh AK, Singh I. The role of neutral sphingomyelinase produced ceramide in lipopolysaccharide-mediated expression of inducible nitric oxide synthase. J Neurochem. 2004; 88:583–593.
Article8. Modur V, Zimmerman GA, Prescott SM, McIntyre TM. Endothelial cell inflammatory responses to tumor necrosis factor alpha. Ceramide-dependent and -independent mitogen-activated protein kinase cascades. J Biol Chem. 1996; 271:13094–13102.9. Bhunia AK, Arai T, Bulkley G, Chatterjee S. Lactosylceramide mediates tumor necrosis factor-alpha-induced intercellular adhesion molecule-1 (ICAM-1) expression and the adhesion of neutrophil in human umbilical vein endothelial cells. J Biol Chem. 1998; 273:34349–34357.10. Chatterjee S. Sphingolipids in atherosclerosis and vascular biology. Arterioscler Thromb Vasc Biol. 1998; 18:1523–1533.
Article11. Tessitore A, Pastore L, Rispoli A, Cilenti L, Toniato E, Flati V, Farina AR, Frati L, Gulino A, Martinotti S. Two gamma-interferon-activation sites (GAS) on the promoter of the human intercellular adhesion molecule (ICAM-1) gene are required for induction of transcription by IFN-gamma. Eur J Biochem. 1998; 258:968–975.
Article12. Lee SJ, Park JY, Hou J, Benveniste EN. Transcriptional regulation of the intercellular adhesion molecule-1 gene by proinflammatory cytokines in human astrocytes. Glia. 1999; 25:21–32.
Article13. Park ES, You SH, Kim H, Kwon OY, Ro HK, Cho BY, Taniguchi SI, Kohn LD, Shong M. Hormone-dependent regulation of intercellular adhesion molecule-1 gene expression: cloning and analysis of 5′-regulatory region of rat intercellular adhesion molecule-1 gene in FRTL-5 rat thyroid cells. Thyroid. 1999; 9:601–612.
Article14. Hurwitz AA, Lyman WD, Guida MP, Calderon TM, Berman JW. Tumor necrosis factor alpha induces adhesion molecule expression on human fetal astrocytes. J Exp Med. 1992; 176:1631–1636.
Article15. Kałuza J, Krupiński J, Kumar P, Kumar S, Wang JM. VCAM-1 expression on reactive and tumour astrocytes. Folia Histochem Cytobiol. 1994; 32:17–20.16. Héry C, Sébire G, Peudenier S, Tardieu M. Adhesion to human neurons and astrocytes of monocytes: the role of interaction of CR3 and ICAM-1 and modulation by cytokines. J Neuroimmunol. 1995; 57:101–109.
Article17. Vitolo D, Paradiso P, Uccini S, Ruco LP, Baroni CD. Expression of adhesion molecules and extracellular matrix proteins in glioblastomas: relation to angiogenesis and spread. Histopathology. 1996; 28:521–528.
Article18. Lee JH, Kim CH, Seo GH, Lee J, Kim JH, Kim DG, Ahn YS. Heparin attenuates the expression of TNF alpha-induced cerebral endothelial cell adhesion molecule. Korean J Physiol Pharmacol. 2008; 12:231–236.19. Ginis I, Schweizer U, Brenner M, Liu J, Azzam N, Spatz M, Hallenbeck JM. TNF-alpha pretreatment prevents subsequent activation of cultured brain cells with TNF-alpha and hypoxia via ceramide. Am J Physiol. 1999; 276:C1171–1183.20. Chang YJ, Holtzman MJ, Chen CC. Interferon-gamma-induced epithelial ICAM-1 expression and monocyte adhesion. Involvement of protein kinase C-dependent c-Src tyrosine kinase activation pathway. J Biol Chem. 2002; 277:7118–7426.21. Park EJ, Ji KA, Jeon SB, Choi WH, Han IO, You HJ, Kim JH, Jou I, Joe EH. Rac1 contributes to maximal activation of STAT1 and STAT3 in IFN-gamma-stimulated rat astrocytes. J Immunol. 2004; 173:5697–5703.22. Qin H, Niyongere SA, Lee SJ, Baker BJ, Benveniste EN. Expression and functional significance of SOCS-1 and SOCS-3 in astrocytes. J Immunol. 2008; 181:3167–3176.
Article23. Ahmad M, Theofanidis P, Medford RM. Role of activating protein-1 in the regulation of the vascular cell adhesion molecule-1 gene expression by tumor necrosis factor-alpha. J Biol Chem. 1998; 273:4616–4621.24. Lee SJ, Hou J, Benveniste EN. Transcriptional regulation of intercellular adhesion molecule-1 in astrocytes involves NF-kappaB and C/EBP isoforms. J Neuroimmunol. 1998; 92:196–207.25. Chen C, Chou C, Sun Y, Huang W. Tumor necrosis factor alpha-induced activation of downstream NF-kappaB site of the promoter mediates epithelial ICAM-1 expression and monocyte adhesion. Involvement of PKCalpha, tyrosine kinase, and IKK2, but not MAPKs, pathway. Cell Signal. 2001; 13:543–553.26. Lee YW, Kühn H, Hennig B, Neish AS, Toborek M. IL-4-induced oxidative stress upregulates VCAM-1 gene expression in human endothelial cells. J Mol Cell Cardiol. 2001; 33:83–94.
Article27. Lazzerini G, Del Turco S, Basta G, O'Loghlen A, Zampolli A, Caterina RD. Prominent role of NF-kappaB in the induction of endothelial activation by endogenous nitric oxide inhibition. Nitric Oxide. 2009; 21:184–191.28. Yeh LH, Kinsey AM, Chatterjee S, Alevriadou BR. Lactosylceramide mediates shear-induced endothelial superoxide production and intercellular adhesion molecule-1 expression. J Vasc Res. 2001; 38:551–559.
Article29. Pastores GM, Giraldo P, Chérin P, Mehta A. Goal-oriented therapy with miglustat in Gaucher disease. Curr Med Res Opin. 2009; 25:23–37.
Article30. Huang WC, Tsai CC, Chen CL, Chen TY, Chen YP, Lin YS, Lu PJ, Lin CM, Wang SH, Tsao CW, Wang CY, Cheng YL, Hsieh CY, Tseng PC, Lin CF. Glucosylceramide synthase inhibitor PDMP sensitizes chronic myeloid leukemia T315I mutant to Bcr-Abl inhibitor and cooperatively induces glycogen synthase kinase-3-regulated apoptosis. FASEB J. 2011; 25:3661–3673.
Article31. Baran Y, Bielawski J, Gunduz U, Ogretmen B. Targeting glucosylceramide synthase sensitizes imatinib-resistant chronic myeloid leukemia cells via endogenous ceramide accumulation. J Cancer Res Clin Oncol. 2011; 137:1535–1544.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Regulation of TNF - alpha Gene Expression in Human Fetal Astrocytes
- Activation of PPARalpha Attenuates IFNgamma and IL-1beta-induced Cell Proliferation in Astrocytes: Involvement of IL-6 Independent Pathway
- Heparin Attenuates the Expression of TNF alpha-induced Cerebral Endothelial Cell Adhesion Molecule
- Effect of Ultraviolet Light on the Expression of Adhesion Molecules and T Lymphocyte Adhesion to Human Dermal Microvascular Endothelial Cells
- Effect of cytokines on the expression of cell adhesion molecule and on the adhesion of melanoma cells to endothelial cells