Korean J Physiol Pharmacol.
2009 Jun;13(3):241-249. 10.4196/kjpp.2009.13.3.241.
Role of T-type Ca2+ Channels in the Spontaneous Phasic Contraction of Pregnant Rat Uterine Smooth Muscle
- Affiliations
-
- 1Department of Physiology, BK 21 Project for Medical Sciences, College of Medicine, Yonsei University, Seoul 120-752, Korea. yhlee@yumc.yonsei.ac.kr
- KMID: 2071677
- DOI: http://doi.org/10.4196/kjpp.2009.13.3.241
Abstract
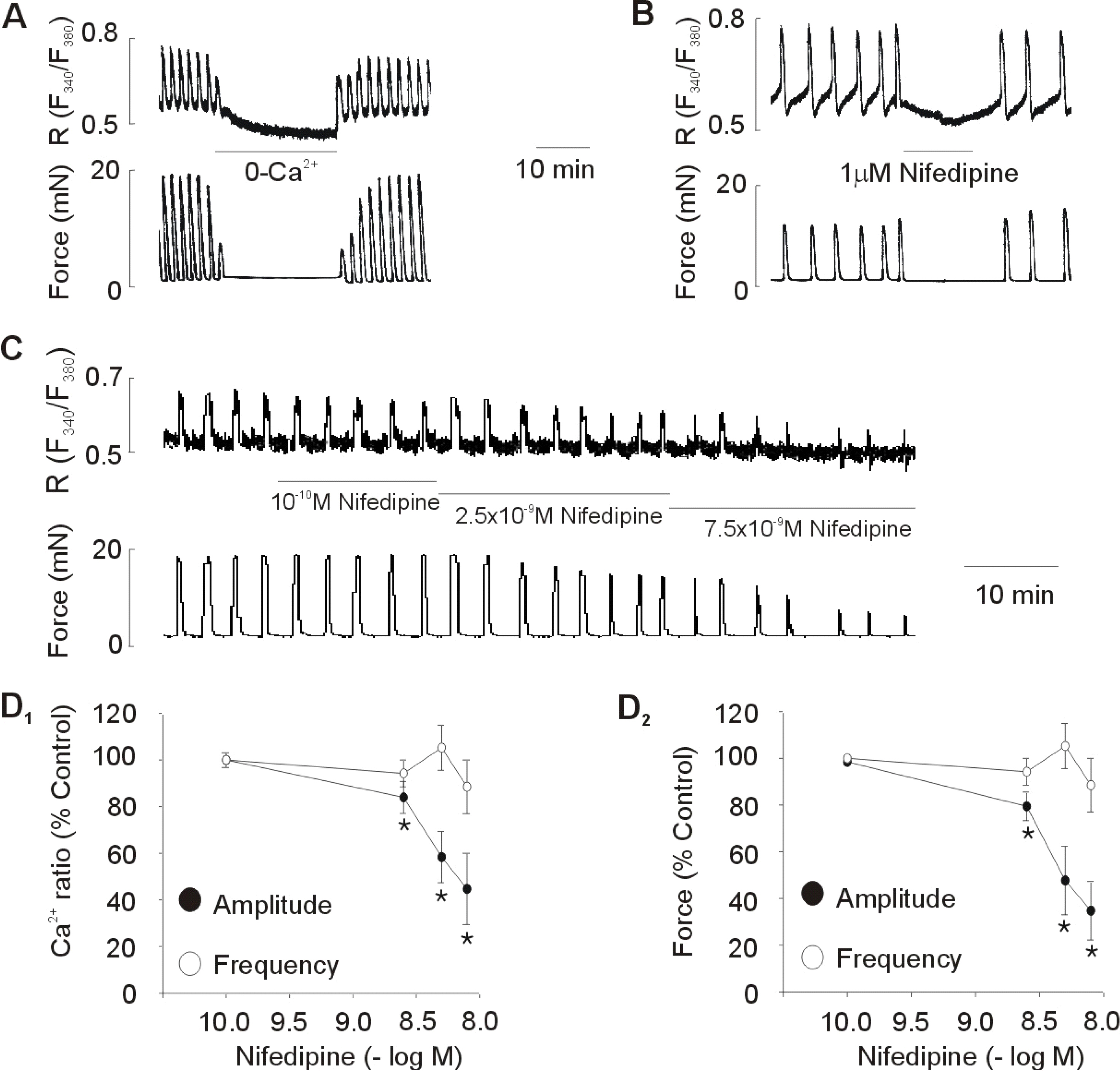
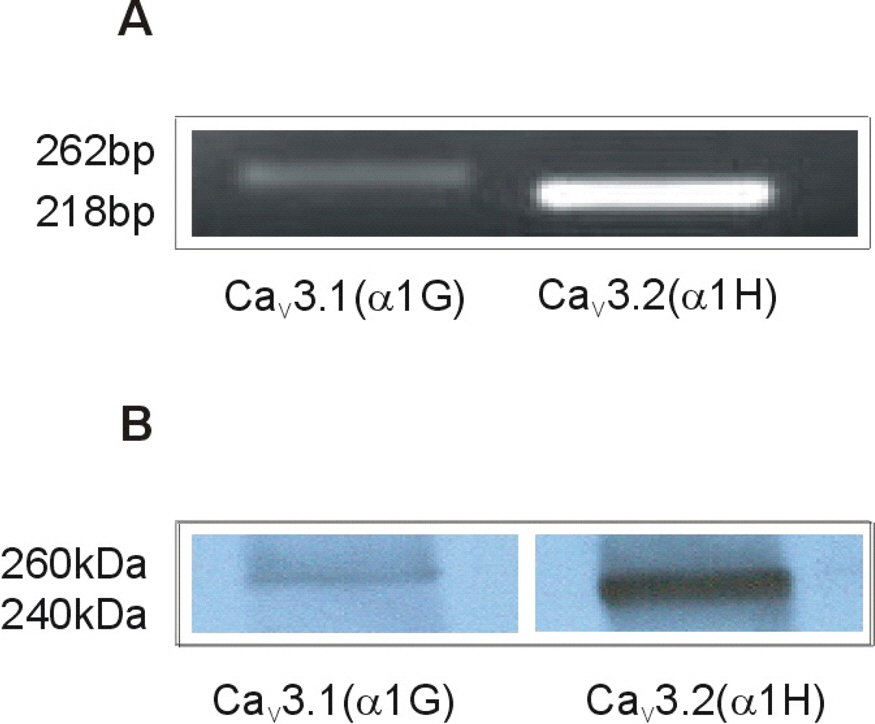
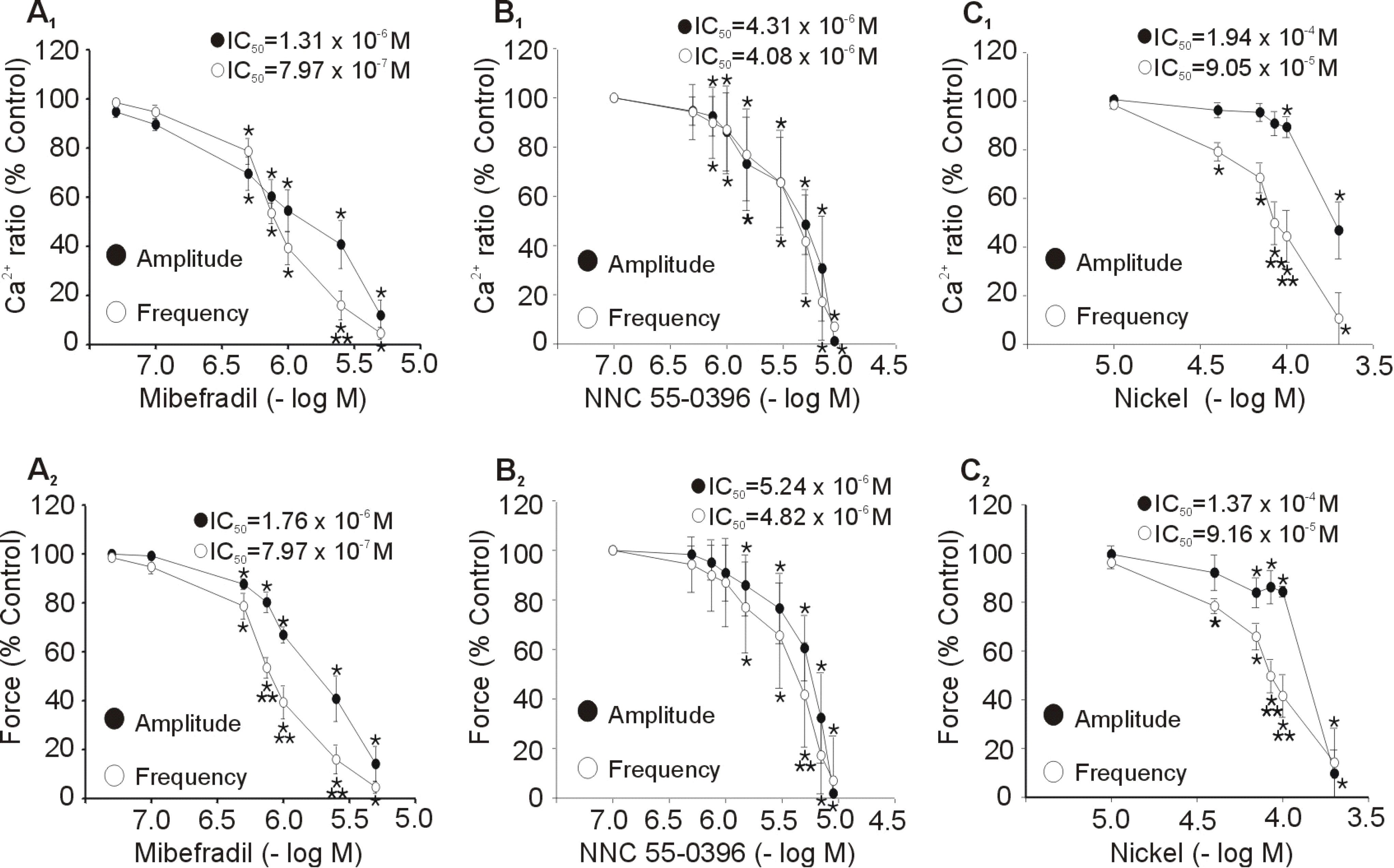
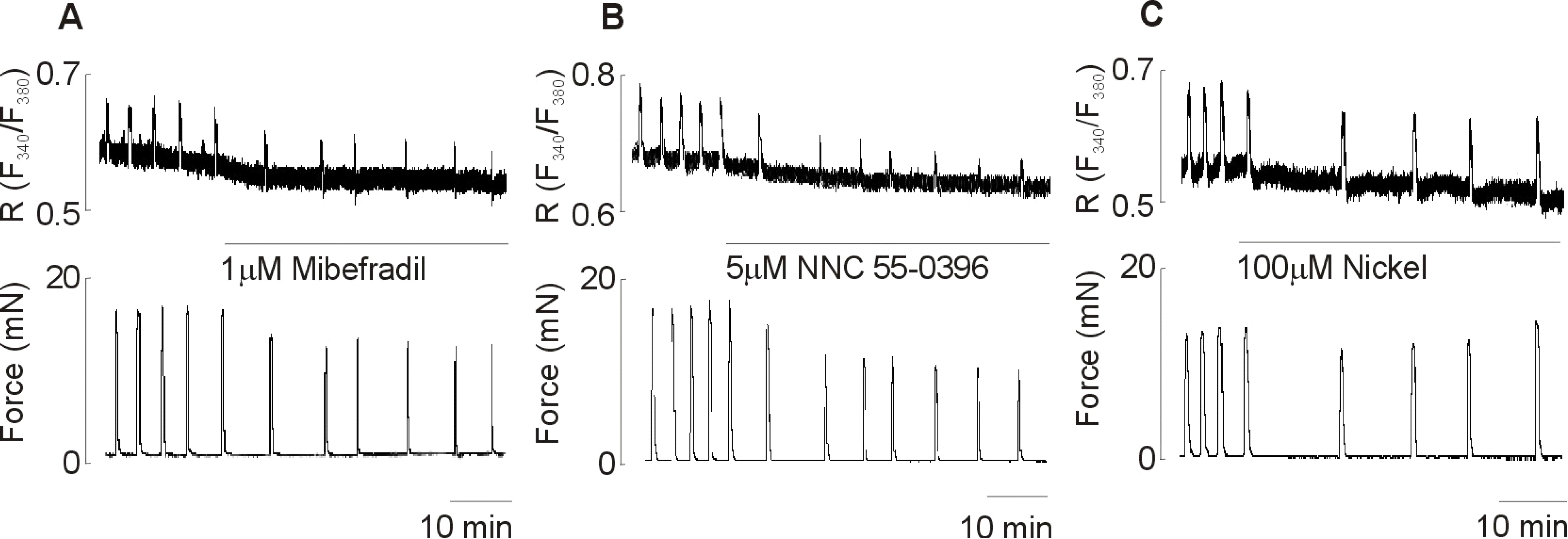
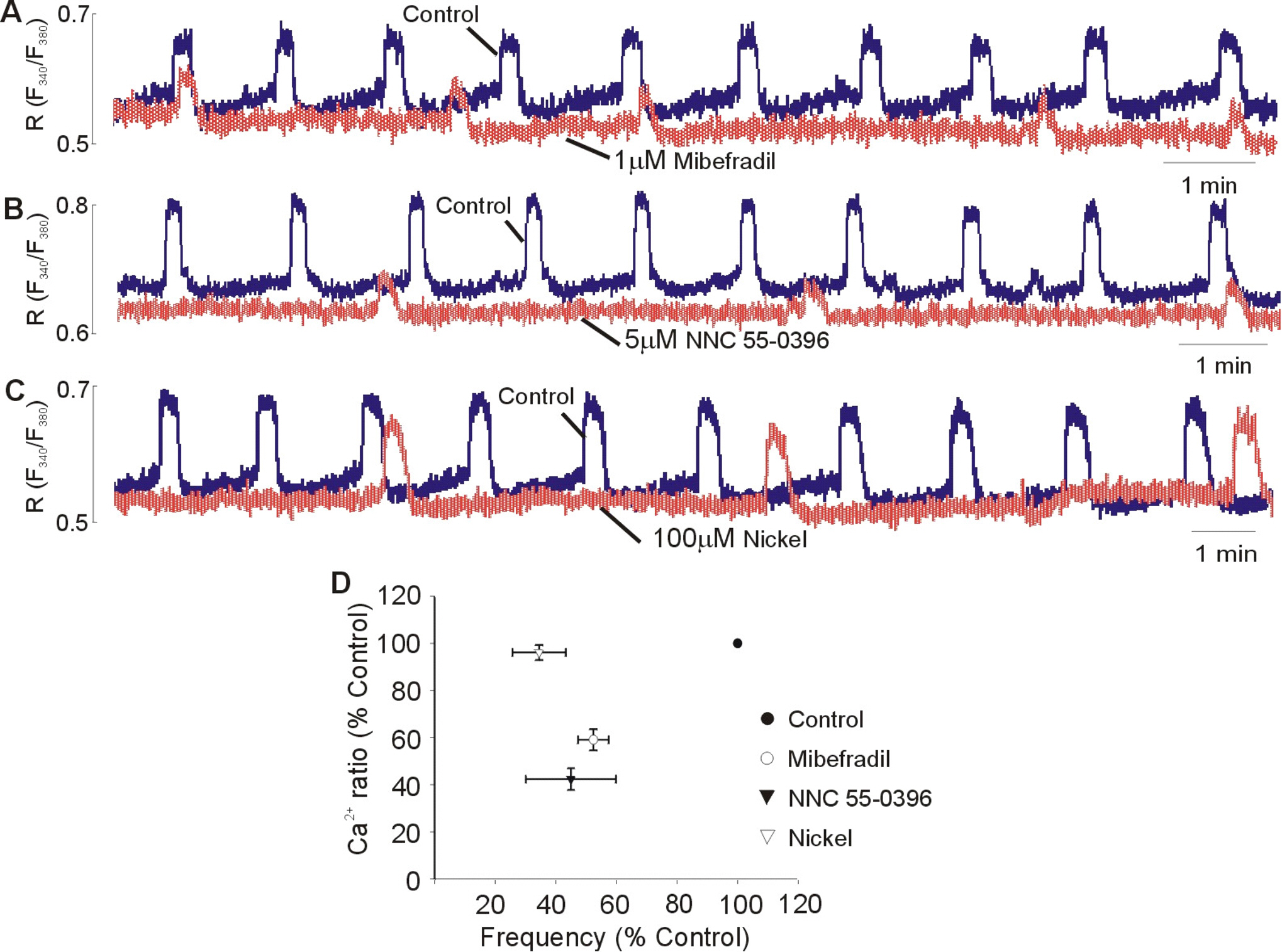
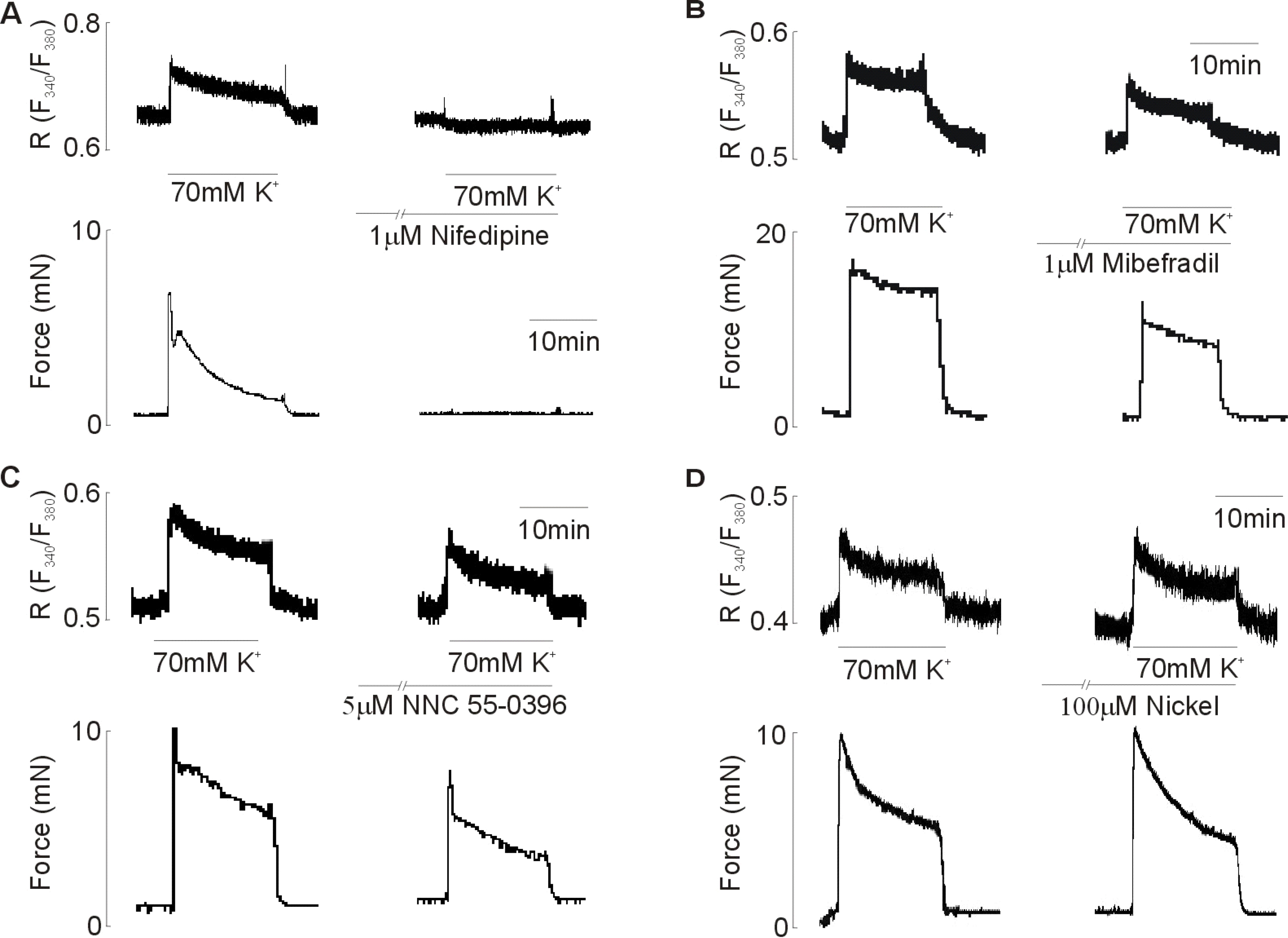
- Although extracellular Ca2+ entry through the voltage-dependent Ca2+ channels plays an important role in the spontaneous phasic contractions of the pregnant rat myometrium, the role of the T-type Ca2+ channels has yet to be fully identified. The aim of this study was to investigate the role of the T-type Ca2+ channel in the spontaneous phasic contractions of the rat myometrium. Spontaneous phasic contractions and [Ca2+]i were measured simultaneously in the longitudinal strips of female Sprague-Dawley rats late in their pregnancy (on day 18~20 of gestation: term=22 days). The expression of T-type Ca2+ channel mRNAs or protein levels was measured. Cumulative addition of low concentrations (<1 micrometer) of nifedipine, a L-type Ca2+ channel blocker, produced a decrease in the amplitude of the spontaneous Ca2+ transients and contractions with no significant change in frequency. The mRNAs and proteins encoding two subunits (alpha1G, alpha1H) of the T-type Ca2+ channels were expressed in longitudinal muscle layer of rat myometrium. Cumulative addition of mibefradil, NNC 55-0396 or nickel induced a concentration-dependent inhibition of the amplitude and frequency of the spontaneous Ca2+ transients and contractions. Mibefradil, NNC 55-0396 or nickel also attenuated the slope of rising phase of spontaneous Ca2+ transients consistent with the reduction of the frequency. It is concluded that T-type Ca2+ channels are expressed in the pregnant rat myometrium and may play a key role for the regulation of the frequency of spontaneous phasic contractions.
MeSH Terms
-
Animals
Benzimidazoles
Calcium Channels
Contracts
Cyclopropanes
Female
Humans
Mibefradil
Mice
Muscle, Smooth
Muscles
Myometrium
Naphthalenes
Nickel
Nifedipine
Pregnancy
Proteins
Rats
Rats, Sprague-Dawley
RNA, Messenger
Benzimidazoles
Calcium Channels
Cyclopropanes
Mibefradil
Naphthalenes
Nickel
Nifedipine
Proteins
RNA, Messenger
Figure
Reference
-
Asokan KT., Sarkar SN., Mishra SK., Raviprakash V. Effects of mibefradil on uterine contractility. Eur J Pharmacol. 455:65–71. 2002.
ArticleBillman GE. Ro 40-5967, a novel calcium channel antagonist, protects against ventricular fibrillation. Eur J Pharmacol. 229:179–187. 1992.
ArticleBlanks AM., Zhao ZH., Shmygol A., Bru-Mercier G., Astle S., Thornton S. Characterization of the molecular and electrophysiological properties of the T-type calcium channel in human myometrium. J Physiol. 581(Pt 3):915–926. 2007.
ArticleChallis JRG., Matthews SG., Gibb W., Lye SJ. Endocrine and paracrine regulation of birth at term and preterm. Endocrine Rev. 21:514–550. 2000.
ArticleChien EK., Saunders T., Phillippe M. The mechanisms underlying Bay K 8644-stimulated phasic myometrial contractions. J Soc Gynecol Invest. 3:106–112. 1996.
ArticleChow KY., Wu C., Sui GP., Fry CH. Role of the T-type Ca2+ current on the contractile performance of guinea pig detrusor smooth muscle. Neurourol Urodyn. 22:77–82. 2003.Coleman HA., Hart JDE., Tonta MA., Parkington HC. Changes in the mechanisms involved in uterine contractions during pregnancy in guinea-pigs. J Physiol. 523:785–798. 2000.
ArticleColeman HA., Parkington HC. The role of membrane potential in the control of uterine motility. Carsten ME, Miller JD, editors. ed,. Uterine function: Molecular and cellular aspects. New York: Plenum Press;p. p. 195–248. 1990.Collins PL., Moore JJ., Idriss E., Kulp TM. Human fetal membranes inhibit calcium L-channel activated uterine contractions. Am J Obstet Gynecol. 175:1173–1179. 1996.
ArticleDoerr T., Denger R., Trautwein W. Calcium currents in single SA nodal cells of the rabbit heart studied with action potential clamp. Pflugers Arch. 413:599–603. 1989.
ArticleHonore E., Amedee T., Martin C., Dacquet C., Mironneau C., Mironneau J. Calcium channel current and its sensitivity to (+) isradipine in cultured pregnant rat myometrial cells. Pflugers Arch. 414:477–483. 1989.Huang L., Keyser BM., Tagmose TM., Hansen JB., Taylor JT., Zhuang H., Zhang M., Ragsdale DS., Li M. NNC 55-0396[(1S,2S)-2-(2-(N-[(3-benzimidazol-2-yl)propyl]-N-methylamino)ethyl)-6-fluoro-1,2,3,4-tetrahydro-1-isopropyl-2-naphytyl cyclopropanecarboxylate dihydrochloride]: a new selective inhibitor of T-type calcium channels. J Pharmacol Exp Therap. 309:193–199. 2004.Inoue Y., Sperelakis N. Gestational change in Na+ and Ca2+ current densities in rat myometrial smooth muscle cells. Am J Physiol. 260:C658–C663. 1991.Jmari K., Mironneau C., Mironneau J. Inactivation of calcium channels current in rat uterine smooth muscle: evidence for calcium and voltage-mediated mechanisms. J Physiol. 380:111–126. 1986.Knock GA., Aaronson PI. Calcium antagonistic properties of the cyclooxygenase-2 inhibitor nimesulide in human myometrial myocytes. Br J Pharmacol. 127:1470–1478. 1999.
ArticleLee JH., Gomora JC., Cribbs LL., Perez-Reyes E. Nickel block of three cloned T-type calcium channels: low concentrations selectively block α1H. Biophys J. 77:3034–3042. 1999.
ArticleMershon JL., Mikala G., Schwartz A. Changes in the expression of the L-type voltage-dependent calcium channel during pregnancy and parturition in the rat. Biol Reprod. 51:993–999. 1994.Mironneau J. Excitation-contraction coupling in voltage clamped uterine smooth muscle. J Physiol. 233:127–141. 1973.
ArticleMishra SK., Hermsmeyer K. Selective inhibition of T-type Ca2+ channels by Ro 40-5967. Circ Res. 75:144–148. 1994.Ohkubo T., Kawarabayashi T., Inoue Y., Kitamura K. Differential expression of L-and T-type calcium channels between longitudinal and circular muscles of the rat myometrium during pregnancy. Gynecol Obstet Invest. 59:80–85. 2005.Ohya Y., Sperelakis N. Fast Na+ and slow Ca2+ channels in single uterine muscle cells from pregnant rats. Am. J Physiol. 257:C408–C412. 1989.Parkington HC., Coleman HA. Ionic mechanisms underlying action potentials in myometrium. Clin Exp Pharmacol Physiol. 15:657–665. 1988.
ArticleParkington HC., Coleman HA. Excitability in uterine smooth muscle. Front Horm Res. 27:179–200. 2001.
ArticleParkington HC., Tonta MA., Brennecke SP., Coleman HA. Contractile activity, membrane potential, and cytoplasmic calcium in human uterine smooth muscle in the third trimester of pregnancy and during labor. Am J Obstet Gynecol. 181:1145–1151. 1999.
ArticlePerez-Reyes E. Molecular physiology of low-voltage-activated T-type calcium channels. Physiol Rev. 83:117–161. 2003.
ArticleRiemer RK., Heymann MA. Regulation of uterine smooth muscle function during gestation. Pediatrics Res. 44:615–627. 1998.
ArticleShmigol AV., Eisner DA., Wray S. Properties of voltage-activated [Ca2+]i transients in single smooth muscle cells isolated from pregnant rat uterus. J Physiol. 511:803–811. 1998.Sui GP., Wu C., Fry CH. Inward calcium currents in cultured and freshly isolated detrusor muscle cells: evidence of a T-type calcium current. J Urol. 165:621–626. 2001.Taggart MJ., Tribe RM. Cellular ionic mechanisms controlling uterine smooth muscle contraction: effects of gestational state. Savineau JP, editor. ed,. New Frontiers in Smooth Muscle Biology and Physiology. India: Research Signpost;p. p. 523–549. 2007.Tytgat J., Vereecke J., Carmeliet E. Combined study of sodium current and T-type calcium current in isolated cardiac cells. Pflugers Arch. 417:142–148. 1990.Wray S., Jones K., Kupittayanant S., Li Y., Matthew A., Monir-Bishty E., Noble K., Pierce SJ., Quenby S., Shmygol AV. Calcium signaling and uterine contractility. J Soc Gynecol Invest. 10:252–264. 2003.Yeon DS., Kim JS., Ahn DS., Kwon SC., Kang BS., Morgan KG., Lee YH. Role of protein kinase C- or RhoA-induced Ca2+ sensitization in stretch-induced myogenic tone. Cardiovas Res. 53:431–438. 2002.Young RC., Smith LH., McLaren MD. T-type and L-type calcium currents in freshly dispersed human uterine smooth muscle cells. Am J Obstet Gynecol. 169:785–792. 2003.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of Opioid Agonists , Diazepam and Ketamine on the Estrous and Pregnant Rat Uteri , in Vitro
- The Effects of Ketamine on Rat Myometrial Contractility
- The relaxant effect of nicardipine on the isolated uterine smooth muscle of the pregnant rat
- Role of Protein Kinase in Spontaneous Phasic Contraction of Cat Gastric Smooth Muscle
- The effects of etomidate on the contraction of pregnant rat uterine smooth muscle