Korean J Physiol Pharmacol.
2009 Jun;13(3):169-173. 10.4196/kjpp.2009.13.3.169.
Immunomodulatory Activity of Ginsan, a Polysaccharide of Panax Ginseng, on Dendritic Cells
- Affiliations
-
- 1Laboratory of Veterinary Pharmacology, College of Veterinary Medicine, Jeju National University, Jeju 690-756, Korea. jooh@jejunu.ac.kr
- 2Applied Radiological Science Research Institute, Jeju National University, Jeju 690-756, Korea.
- 3Laboratory of Immunology, College of Veterinary Medicine, Seoul National University, Seoul 151-742, Korea.
- 4Laboratory of Radiation Immunology, Korea Institute of Radiological and Medical Sciences, Seoul 139-706, Korea.
- KMID: 2071667
- DOI: http://doi.org/10.4196/kjpp.2009.13.3.169
Abstract
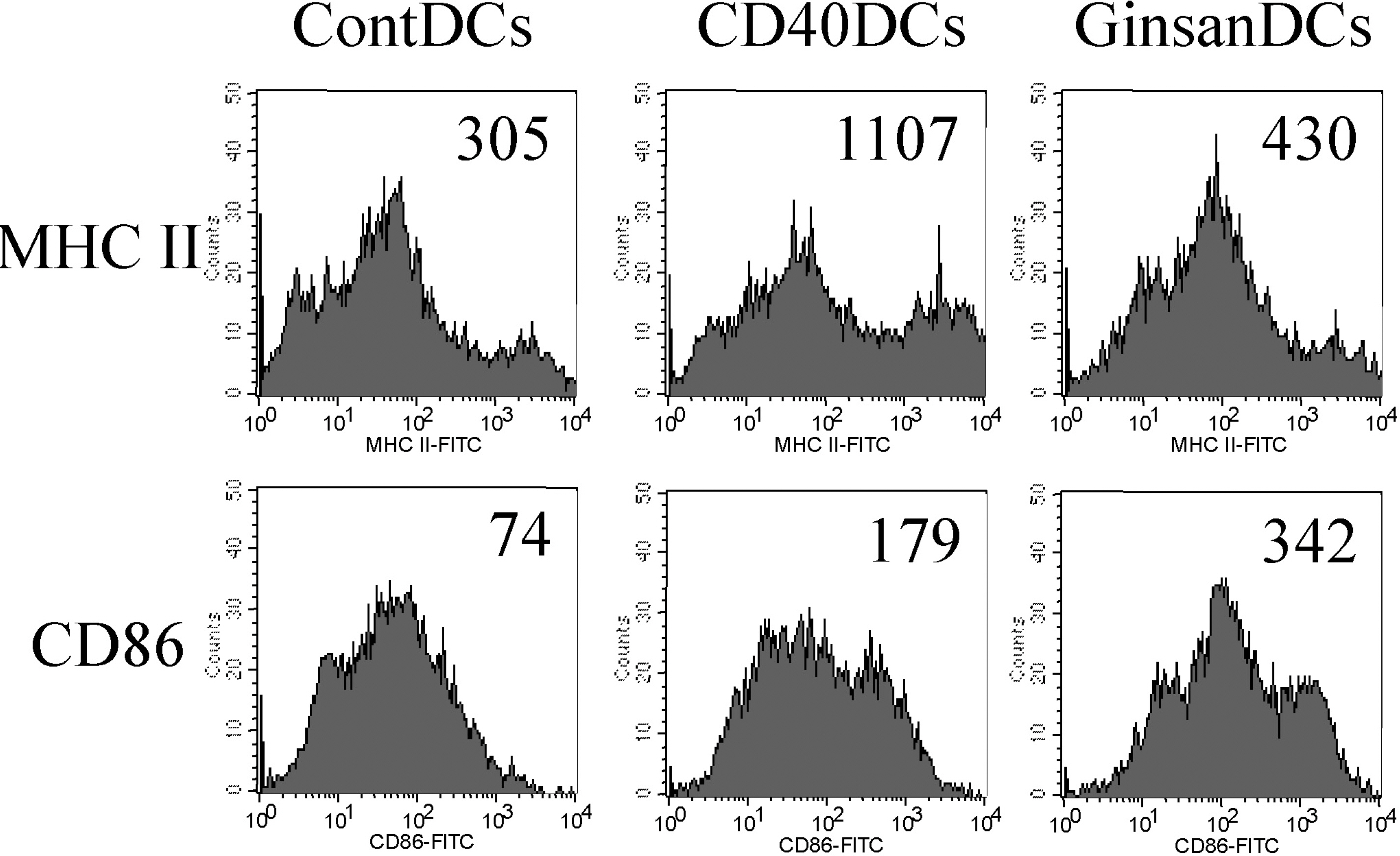
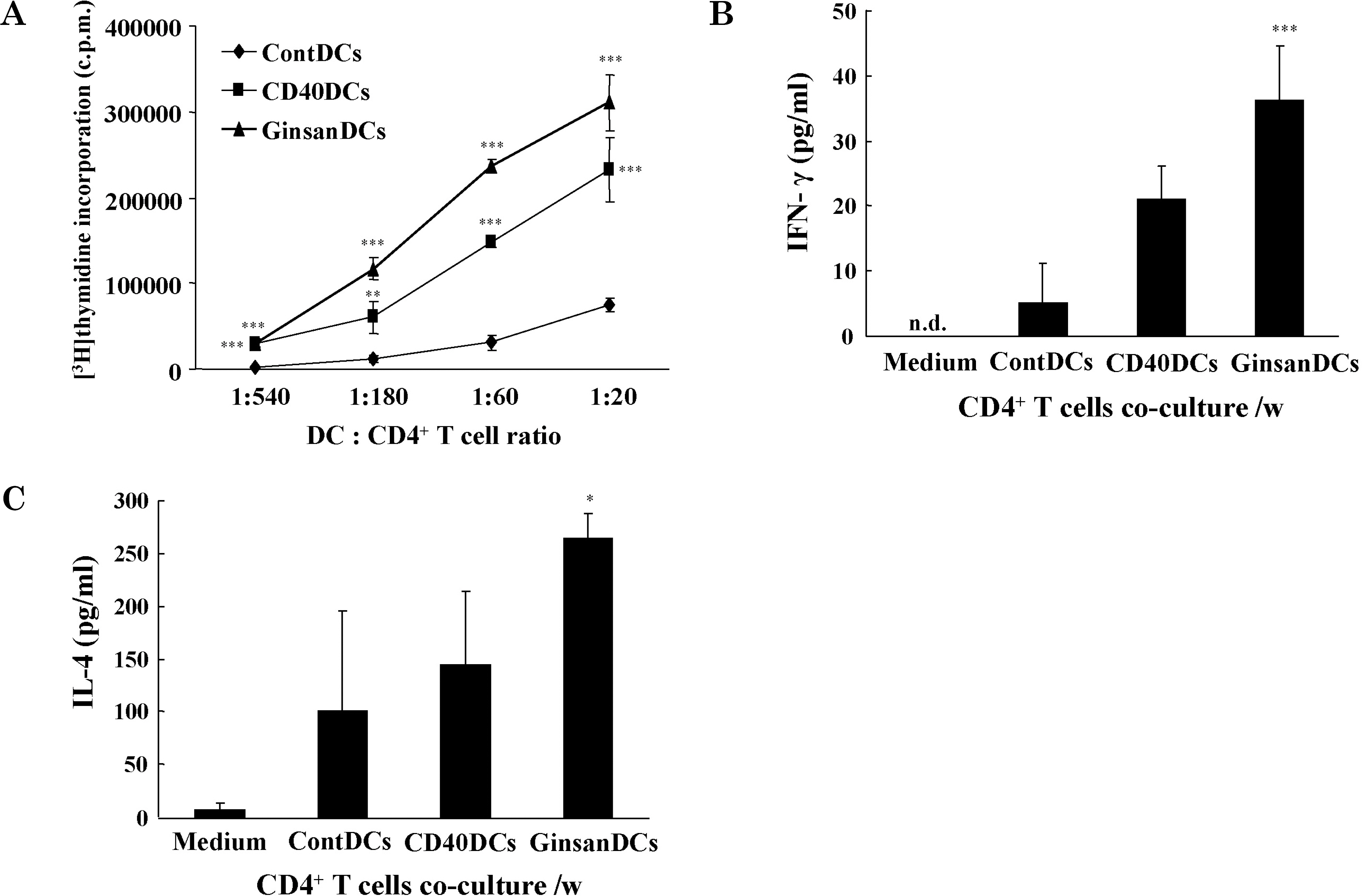
- Ginsan, a Panax ginseng polysaccharide that contains glucopyranoside and fructofuranoside, has immunomodulatory effects. Although several biologic studies of ginsan have been performed, its effects on dendritic cells (DCs), which are antigen-presenting cells of the immune system, have not been studied. We investigated the immunomodulatory effects of ginsan on DCs. Ginsan had little effect on DC viability, even when used at high concentrations. Ginsan markedly increased the levels of production by DCs of IL-12 and TNF-alpha, as measured by ELISA. To examine the maturation-inducing activity of ginsan, we measured the surface expression levels of the maturation markers MHC class II and CD86 (B7.2) on DCs. It is interesting that ginsan profoundly enhanced the expression of CD86 on DC surfaces, whereas it increased that of MHC class II only marginally. In 3H-thymidine incorporation assays, ginsan-treated DCs stimulated significantly higher proliferation of allogeneic CD4+ T lymphocytes than did medium-treated DCs. Taken together, our data demonstrate that ginsan stimulates DCs by inducing maturation. Because DCs are critical antigen-presenting cells in immune responses, this study provides valuable information on the activities of ginsan.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Maturation of bone marrow-derived dendritic cells by a novel β-glucan purified from Paenibacillus polymyxa JB115
Eun-Ju Ko, Yun-Young Byon, Youngheun Jee, Taekyun Shin, Seung-Chun Park, Tae-Wook Hahn, Hong-Gu Joo
J Vet Sci. 2011;12(2):187-189. doi: 10.4142/jvs.2011.12.2.187.
Reference
-
Ahn JY., Choi IS., Shim JY., Yun EK., Yun YS., Jeong G., Song JY. The immunomodulator ginsan induces resistance to experimental sepsis by inhibiting Toll-like receptor-mediated inflammatory signals. Eur J Immunol. 36:37–45. 2006.
ArticleBonham CA., Lu L., Li Y., Hoffman RA., Simmons RL., Thomson AW. Nitric oxide production by mouse bone marrow-derived dendritic cells: implications for the regulation of allogeneic T cell responses. Transplantation. 62:1871–1877. 1996.Corinti S., Albanesi C., la Sala A., Pastore S., Girolomoni G. Regulatory activity of autocrine IL-10 on dendritic cell functions. J Immunol. 166:4312–4318. 2001.
ArticleEsche C., Shurin GV., Kirkwood JM., Wang GQ., Rabinowich H., Pirtskhalaishvili G., Shurin MR. Tumor necrosis factor-alpha-promoted expression of Bcl-2 and inhibition of mitochondrial cytochrome c release mediate resistance of mature dendritic cells to melanoma-induced apoptosis. Clin Cancer Res. 7:974s–979s. 2001.Heufler C., Koch F., Stanzl U., Topar G., Wysocka M., Trinchieri G., Enk A., Steinman RM., Romani N., Schuler G. Interleukin-12 is produced by dendritic cells and mediates T helper 1 development as well as interferon-gamma production by T helper 1 cells. Eur J Immunol. 26:659–668. 1996.Joo HG. Altered maturation of dendritic cells by taxol, an anti-cancer drug. J Vet Sci. 4:229–234. 2003.
ArticleJoo HG., Goedegebuure PS., Sadanaga N., Nagoshi M., von Bernstorff W., Eberlein TJ. Expression and function of galectin-3, a beta-galactoside-binding protein in activated T lymphocytes. J Leukoc Biol. 69:555–564. 2001.Kim HJ., Kim MH., Byon YY., Park JW., Jee Y., Joo HG. Radioprotective effects of an acidic polysaccharide of Panax ginseng on bone marrow cells. J Vet Sci. 8:39–44. 2007.
ArticleKim MH., Joo HG. Immunostimulatory effects of fucoidan on bone marrow-derived dendritic cells. Immunol Lett. 115:138–143. 2008.
ArticleLarsen CP., Ritchie SC., Hendrix R., Linsley PS., Hathcock KS., Hodes RJ., Lowry RP., Pearson TC. Regulation of immunostimulatory function and costimulatory molecule (B7-1 and B7-2) expression on murine dendritic cells. J Immunol. 152:5208–5219. 1994.Lee YS., Chung IS., Lee IR., Kim KH., Hong WS., Yun YS. Activation of multiple effector pathways of immune system by the antineoplastic immunostimulator acidic polysaccharide ginsan isolated from Panax ginseng. Anticancer Res. 17:323–331. 1997.Lu L., Bonham CA., Chambers FG., Watkins SC., Hoffman RA., Simmons RL., Thomson AW. Induction of nitric oxide synthase in mouse dendritic cells by IFN-gamma, endotoxin, and interaction with allogeneic T cells: nitric oxide production is associated with dendritic cell apoptosis. J Immunol. 157:3577–3586. 1996.Mellman I., Steinman RM. Dendritic cells: specialized and regulated antigen processing machines. Cell. 106:255–258. 2001.Mosca PJ., Hobeika AC., Clay TM., Nair SK., Thomas EK., Morse MA., Lyerly HK. A subset of human monocyte-derived dendritic cells expresses high levels of interleukin-12 in response to combined CD40 ligand and interferon-gamma treatment. Blood. 96:3499–3504. 2000.Shin JY., Song JY., Yun YS., Yang HO., Rhee DK., Pyo S. Immunostimulating effects of acidic polysaccharides extract of Panax ginseng on macrophage function. Immunopharmacol Immunotoxicol. 24:469–482. 2002.Song JY., Han SK., Son EH., Pyo SN., Yun YS., Yi SY. Induction of secretory and tumoricidal activities in peritoneal macrophages by ginsan. Int Immunopharmacol. 2:857–865. 2002.
ArticleSong JY., Han SK., Bae KG., Lim DS., Son SJ., Jung IS., Yi SY., Yun YS. Radioprotective effects of ginsan, an immunomodulator. Radiat Res. 159:768–774. 2003.
ArticleSteinman RM., Pack M., Inaba K. Dendritic cells in the T-cell areas of lymphoid organs. Immunol Rev. 156:25–37. 1997.
ArticleYoneyama H., Matsuno K., Matsushima K. Migration of dendritic cells. Int J Hematol. 81:204–207. 2005.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Stimulatory Effects of Ginsan on the Proliferation and Viability of Mouse Spleen Cells
- Comparison between Immunostimulatory Activity and Molecular Structure of Different Polysaccharides
- Ginsan Enhances Humoral Antibody Response to Orally Delivered Antigen
- Radioprotective effects of an acidic polysaccharide of Panax ginseng on bone marrow cells
- Brief Introduction of Panax ginseng C.A. Meyer