Korean J Physiol Pharmacol.
2008 Feb;12(1):25-30. 10.4196/kjpp.2008.12.1.25.
Involvement of Thromboxane A2 in the Modulation of Pacemaker Activity of Interstitial Cells of Cajal of Mouse Intestine
- Affiliations
-
- 1Department of Neurology, College of Medicine, Chosun University, Gwangju 501-759, Korea.
- 2Department of Radiology, Gacheon University Gil Medical Center, Guwol-dong, Incheon 405-760, Korea.
- 3Department of Physiology, College of Medicine, Chosun University, Gwangju 501-759, Korea. jyjun@chosun.ac.kr
- KMID: 2071642
- DOI: http://doi.org/10.4196/kjpp.2008.12.1.25
Abstract
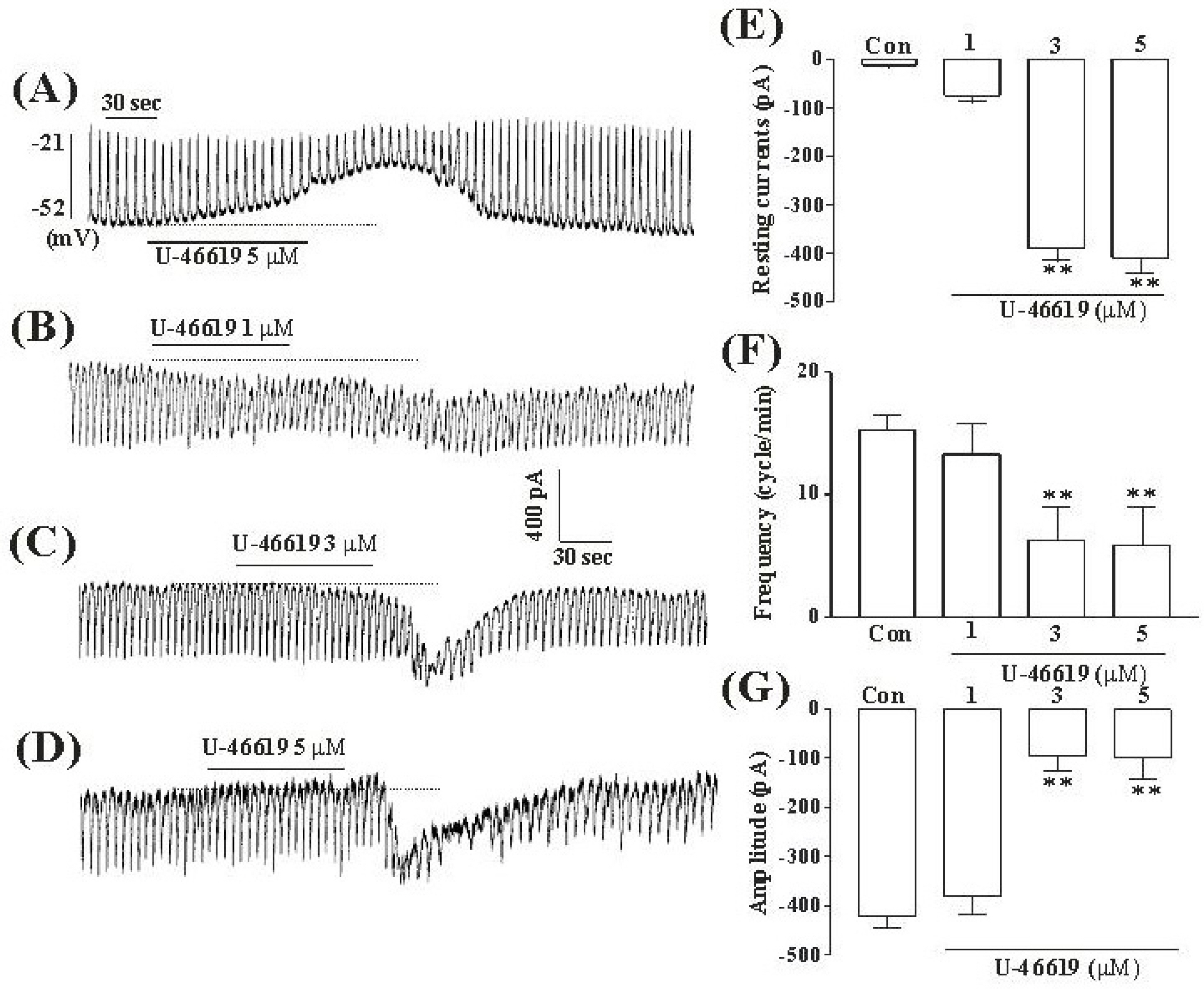
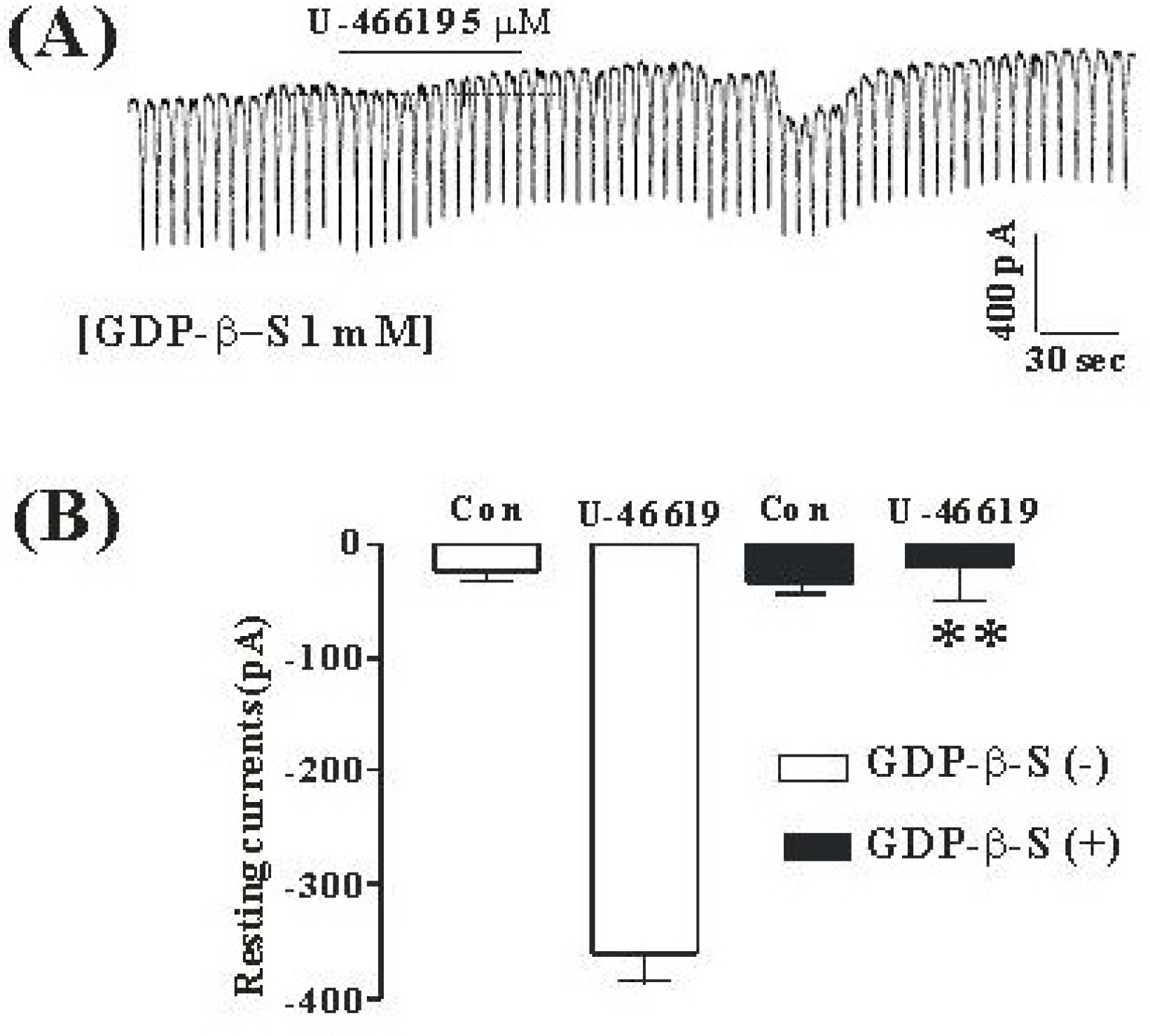
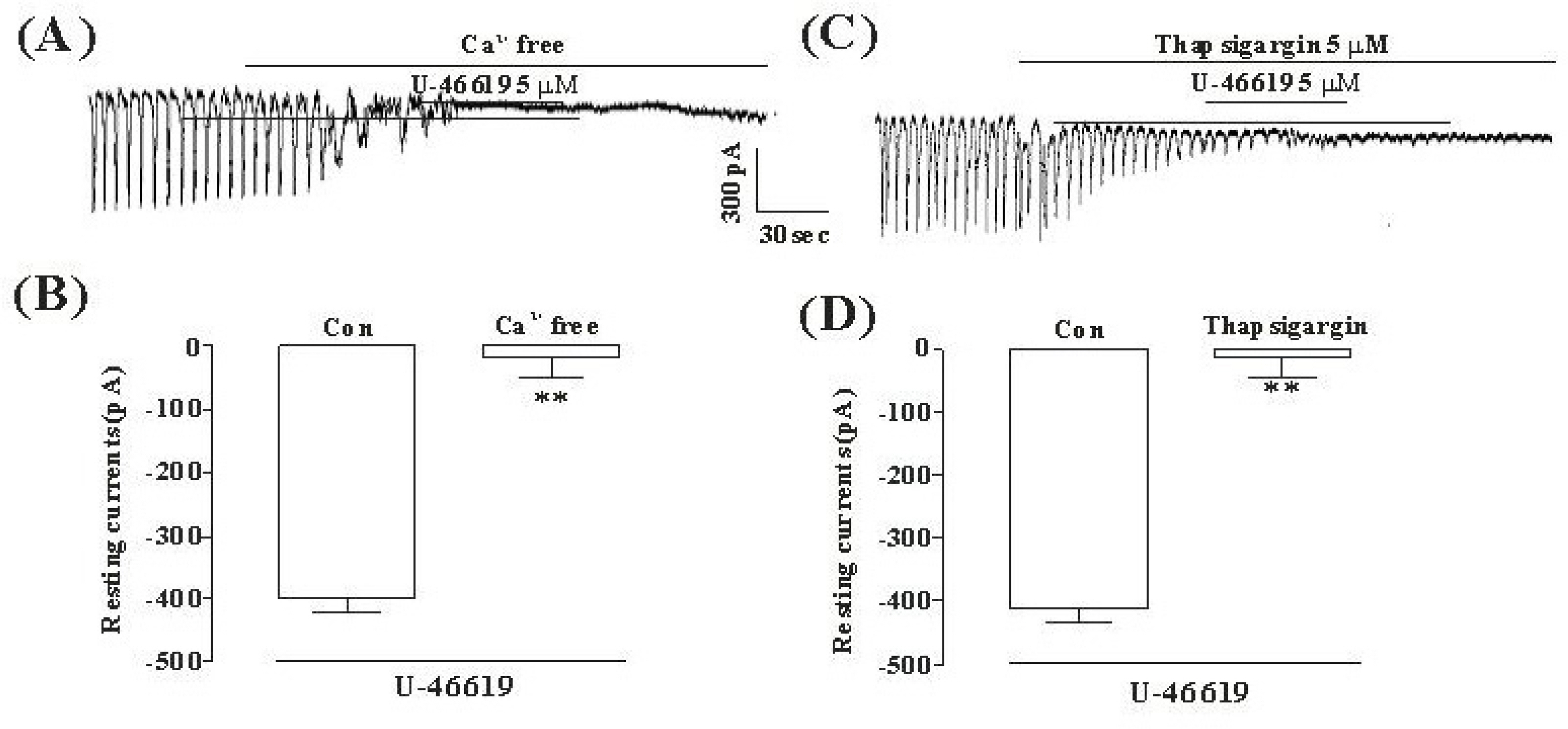
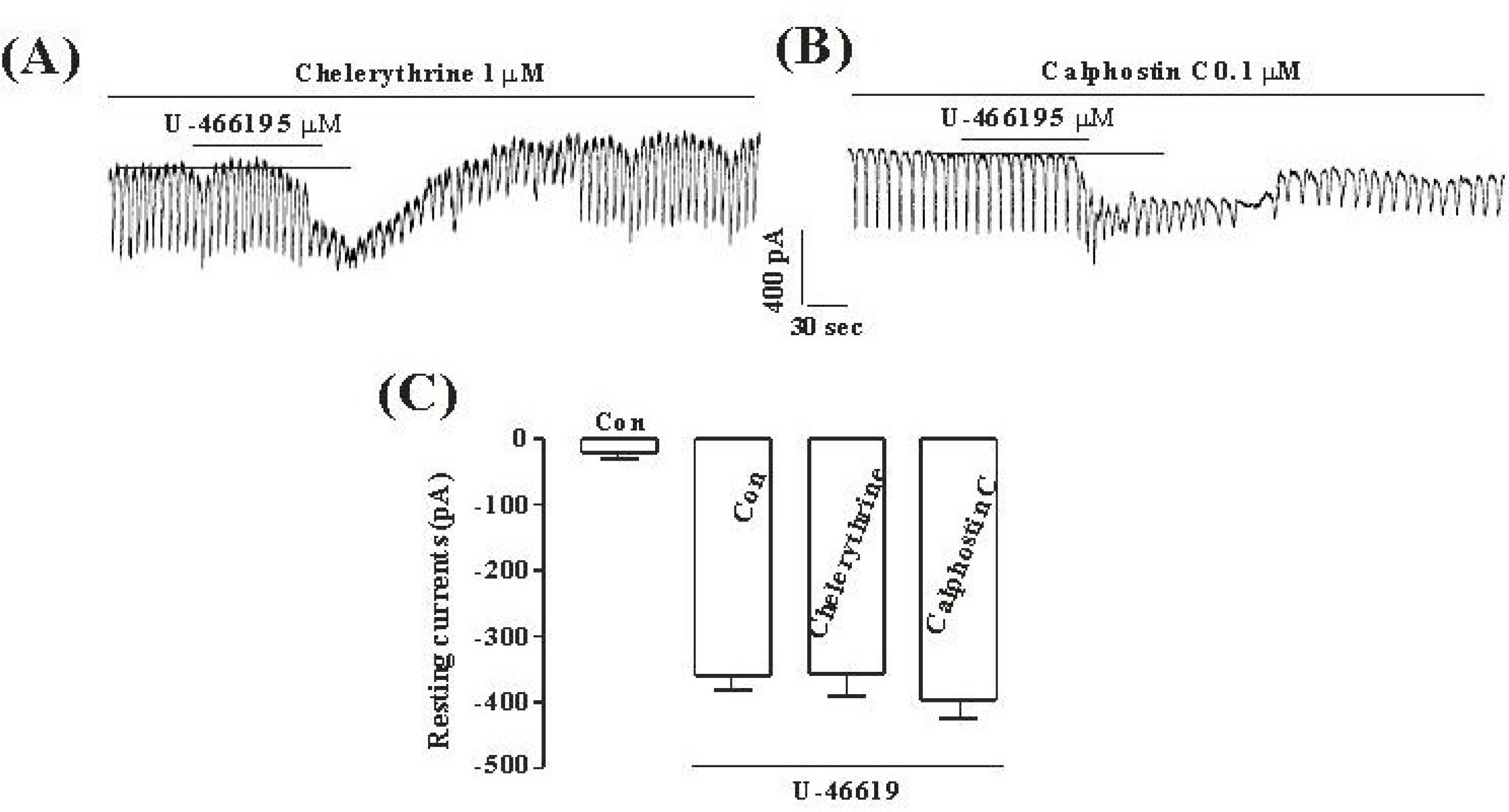
- Although many studies show that thromboxane A2 (TXA2) has the action of gastrointestinal (GI) motility using GI muscle cells and tissue, there are no reports on the effects of TXA2 on interstitial cells of Cajal (ICC) that function as pacemaker cells in GI tract. So, we studied the modulation of pacemaker activities by TXA2 in ICC with whole cell patch-clamp technique. Externally applied TXA2 (5 micrometer) produced membrane depolarization in current-clamp mode and increased tonic inward pacemaker currents in voltage-clamp mode. The tonic inward currents by TXA2 were inhibited by intracellular application of GDP-beta-S. The pretreatment of ICC with Ca2+ free solution and thapsigargin, a Ca2+-ATPase inhibitor in endoplasmic reticulum, abolished the generation of pacemaker currents and suppressed the TXA2-induced tonic inward currents. However, chelerythrine or calphostin C, protein kinase C inhibitors, did not block the TXA2-induced effects on pacemaker currents. These results suggest that TXA2 can regulate intestinal motility through the modulation of ICC pacemaker activities. This modulation of pacemaker activities by TXA2 may occur by the activation of G protein and PKC independent pathway via extra and intracellular Ca2+ modulation.
Keyword
MeSH Terms
-
Animals
Benzophenanthridines
Endoplasmic Reticulum
Gastrointestinal Motility
Gastrointestinal Tract
GTP-Binding Proteins
Guanosine Diphosphate
Interstitial Cells of Cajal
Intestines
Membranes
Mice
Muscle Cells
Naphthalenes
Patch-Clamp Techniques
Protein Kinase C
Thapsigargin
Thionucleotides
Thromboxane A2
Benzophenanthridines
GTP-Binding Proteins
Guanosine Diphosphate
Naphthalenes
Protein Kinase C
Thapsigargin
Thionucleotides
Thromboxane A2
Figure
Reference
-
Arita H., Nakano T., Hanasaki K. Thromboxane A2: its generation and role in platelet activation. Prog Lipid Res. 28:273–301. 1989.Bennett A., Elev KG., Scholes GB. Effects of prostaglandins E1 and E2 on human, guinea pig and rat isolated small intestine. Br J Pharmacol. 34:630–638. 1968.Bennett A., Hensy CN., Sanger GJ., Stamford IF. Metabolites of arachidonic acid formed by human gastrointestinal tissues and their actions on the muscle layers. Br J Pharmacol. 74:435–444. 1981.
ArticleBennett A., Sanger GJ. Pinane thromboxane A2 analogues are non-selective prostanoid antagonists in rat and human stomach muscle. Br J Pharmacol. 77:591–596. 1982.Berezin I., Daniel EE., Huizinga JD. Ultrastructure of interstitial cells of Cajal in the canine distal esophagus. Can J Physiol Pharmacol. 72:1049–1059. 1994.
ArticleBerezin I., Huizinga JD., Daniel EE. Interstitial cells of Cajal in the canine colon: a special communication network at the inner border of the circular muscle. J Comp Neurol. 273:42–51. 1988.
ArticleBrass LF., Shaller CC., Belmonte EJ. Inositol 1,4,5-triphosphate induced granule secretion in platelets, Evidende that the activation of phospholipase C mediated by platelet thromboxane receptors involves a guanine nucleotide binding protein-dependent mechanism distinct from that of thrombin. J Clin Invest. 79:1269–1275. 1987.Coleman RA., Humphrey PP., Kennedy I., Levy GP., Lumley P. Comparison of the actions of U-46619, a prostaglandin H2-analogue, with those of prostaglandin H2 and thromboxane A2 on some isolated smooth muscle preparations. Br J Pharmacol. 73:773–778. 1981.Daniel EE., Posey-Daniel V. Neuromuscular structures in opossum esophagus: role of interstitial cells of Cajal. Am J Physiol. 246:G305–G315. 1984.
ArticleFerreira SH., Herman AG., Vane JR. Prostaglandin production by rabbit isolated jejunum and its relationship to the inherent tone of the preparation. Br J Pharmacol. 56:469–477. 1976.
ArticleKoh SD., Sanders KM., Ward SM. Spontaneous electrical rhythmicity in cultured interstitial cells of Cajal from the mouse small intestine. J Physiol. 513:203–213. 1998.LeDuc LE., Needleman P. Regional localization of prostacyclin and thromboxane synthesis in dog stomach and intestinal tract. J Pharmacol Exp Ther. 211:181–188. 1979.Nakano T., Hanasaki K., Arita H. Different effects of two thromboxane A2/prostaglandin H2 receptor ligand, U46619 and S-14, on rabbit platelets. FEBS Lett. 234:309–312. 1988.Needleman P., Turk J., Jakschik BA., Morrison AR., Lefkowith JB. Arachidonic acid metabolism. Annu Rev Biochem. 55:69–109. 1986.
ArticleOkada Y., Hara A., Ma H., Xiao CY., Takahata O., Kohgo Y., Narumiya S., Ushikubi F. Characterization of prostanoid receptors mediating contraction of the gastric fundus and ileum: studies using mice deficient in prostanoid receptors. Br J Pharmacol. 131:745–755. 2000.
ArticlePollock WK., Armstrong RA., Brydon LJ., Jones RL., Macintyre DE. Thromboxane-induced phosphatide formation in human platelets. Relationship to receptor occupancy and to changes in cytosolic free calcium. Biochem J. 219:833–842. 1984.Portbury AL., Furness JB., Young HM., Southwell BR., Vigna SR. Localization of NK1 receptor immunoreactivity to neurons and interstitial cells of the guinea-pig gastrointestinal tract. J Comp Neurol. 367:342–351. 1996.Publicover NG., Horowitz NN., Sanders KM. Calcium oscillations in freshly dispersed and cultured interstitial cells of from canine colon. Am J Physiol. 262:C589–C597. 1992.Robert A. Prostaglandins and the gastrointestinal tract. Hohnson LR ed, Physiology of the Gastrointestinal Tract. 1st ed.Raven;New York: p. p. 1407–1434. 1981.Sage SO., Rink TJ. The kinetics of changes in intracellular calcium concentration in fura-2-loaded human platelets. J Biol Chem. 262:16364–16369. 1987.
ArticleSanders KM. Evidence that endogenous prostacyclin modulates the electrical and mechanical activities of canine ileal circular muscle. J Gastroenterol. 19:401. 1981.Sanders KM. Evidence that prostaglandins are local regulatory agents in canine ileal circular muscle. Am J Physiol. 246:G361–G371. 1984.
ArticleSanders KM. Ionic mechanisms of electrical rhythmicity in gastrointestinal smooth muscles. Annu Rev Physiol. 54:439–453. 1992.
ArticleShenker A., Goldsmith P., Unson CG., Spiegel AM. The G protein coupled to the thromboxane A2 receptor in human platelets is a member of the novel Gq family. J Biol Chem. 266:9309–9313. 1991.Shuttleworth CWR., Xue C., Ward SM., de Vente J., Sanders KM. Immunohistochemical localization of 3',5'-cyclic guanosine monophosphate in the canine proximal colon: responses to nitric oxide and electrical stimulation of enteric inhibitory neurons. Neuroscience. 56:513–522. 1993.
ArticleSiess W., Stifel M., Binder H., Weber PC. Activation of V1-receptors by vasopressin stimulates inositol phospholipids hydrolysis and arachidonate metabolism in human platelets. Biochem J. 233:83–91. 1986.Sternini C., Su D., Gamp PD., Bunnett NW. Cellular sites of expression of the neurokinin-1 receptor in the rat gastrointestinal tract. J Comp Neurol. 258:531–540. 1995.
ArticleThomsen L., Robinson TL., Lee JCF., Farraway L., Hughes MJH., Andrews DW., Huizinga JD. Interstitial cells of Cajal generate a rhythmic pacemaker current. Nature Med. 4:448–451. 1998.Thuneberg L. Interstitial cells of Cajal: intestinal pacemakers? Adv Anat Embryol Cell Biol. 71:11–30. 1982.Torihashi S., Kobayashi S., Gerthoffer WT., Sanders KM. Interstitial cells in deep muscular plexus of canine small intestine may be specialized smooth muscle cells. Am J Physiol. 265:G638–G645. 1993.
ArticleWatson SP., Reep B., McConnell RT., Lapetina EG. Collagen stimulates [3H]inositol trisphosphate formaton in indomethacin-treated human platelets. Biochem J. 226:831–837. 1985.Young HM., McConalogue K., Fruness JB., de Vente J. Nitric oxide targets in the guinea-pig intestine identified by induction of cyclic GMP immunoreactivity. Neuroscience. 55:583–596. 1993.
ArticleZhou DS., Komuro T. Interstitial cells associated with the deep muscular plexus of the guinea-pig small intestine, with special reference to the interstitial cells of Cajal. Cell Tissue Res. 268:205–216. 1992.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Modulation of Pacemaker Ca2+ Activity by Serotonin in the Cultured Interstitial Cells of the Cajal Clusters Isolated from Mouse Small Intestine
- Capsaicin Inhibits the Spontaneous Pacemaker Activity in Interstitial Cells of Cajal From the Small Intestine of Mouse
- Interplay of Hydrogen Sulfide and Nitric Oxide on the Pacemaker Activity of Interstitial Cells of Cajal from Mouse Small Intestine
- Effects of ATP on Pacemaker Activity of Interstitial Cells of Cajal from the Mouse Small Intestine
- Expression of Bradykinin Receptors and Effects of Bradykinin in the Interstitial Cells of Cajal from Mouse Small Intestine