Korean J Physiol Pharmacol.
2015 Nov;19(6):523-531. 10.4196/kjpp.2015.19.6.523.
Enhancement of GluN2B Subunit-Containing NMDA Receptor Underlies Serotonergic Regulation of Long-Term Potentiation after Critical Period in the Rat Visual Cortex
- Affiliations
-
- 1Department of Physiology, College of Medicine, The Catholic University of Korea, Seoul 06591, Korea. hjjang@catholic.ac.kr
- 2Catholic Neuroscience Institute, The Catholic University of Korea, Seoul 06591, Korea.
- KMID: 2070792
- DOI: http://doi.org/10.4196/kjpp.2015.19.6.523
Abstract
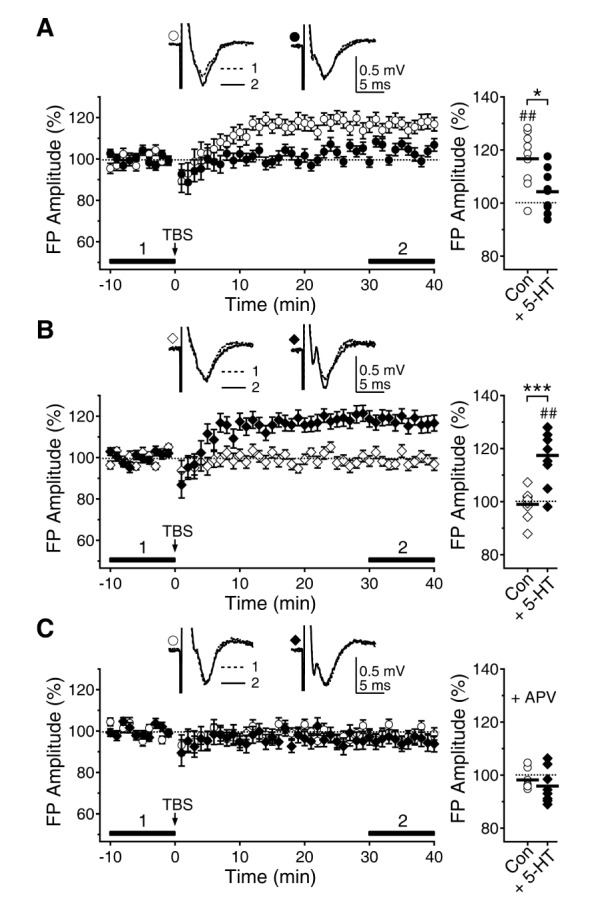
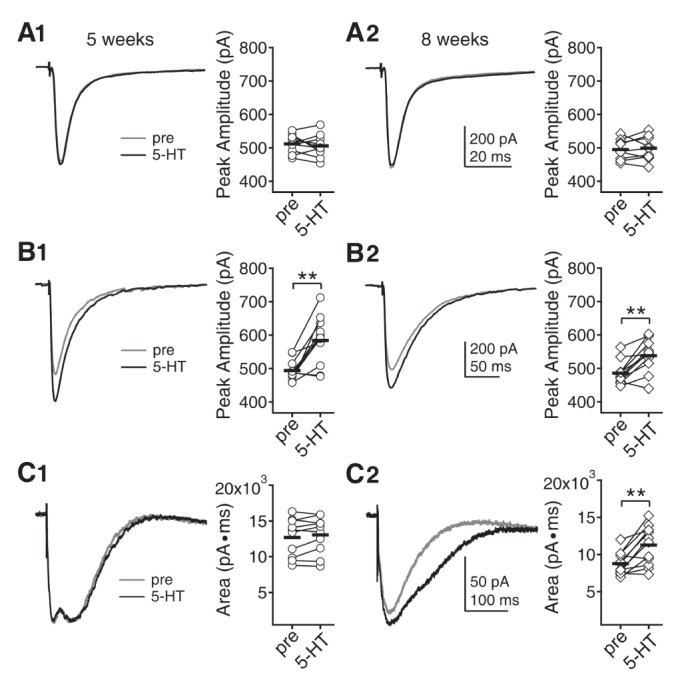
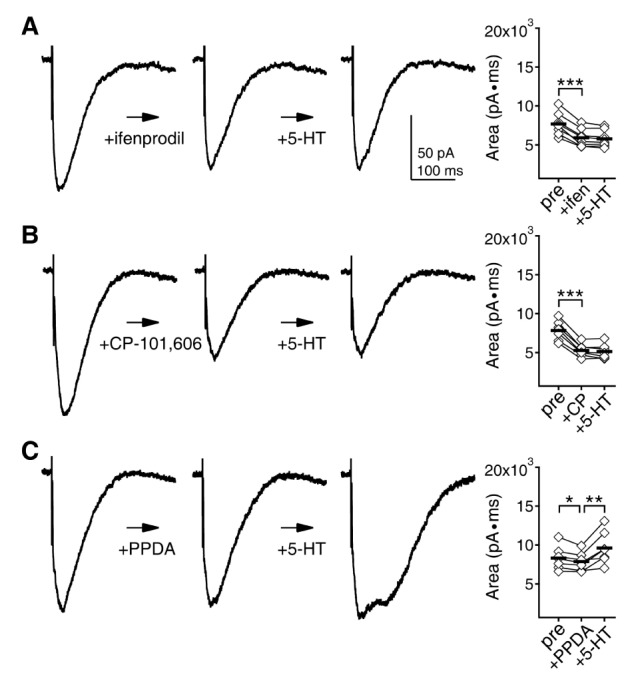
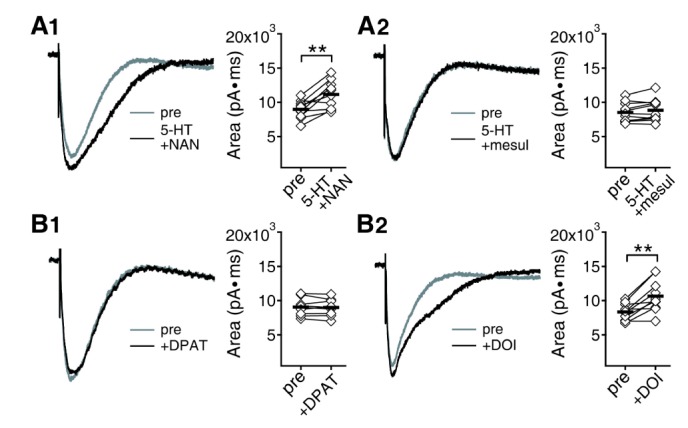
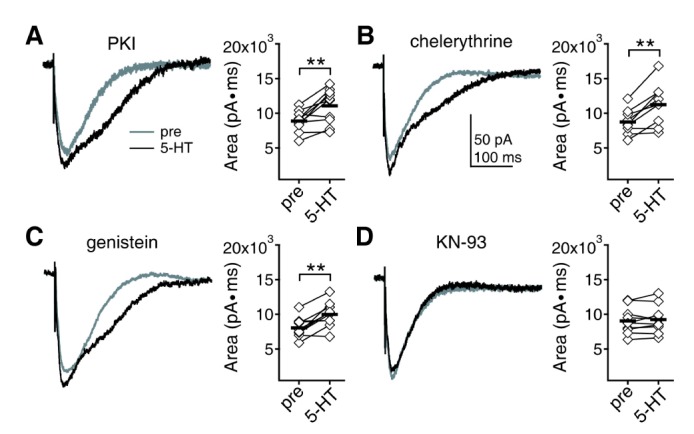
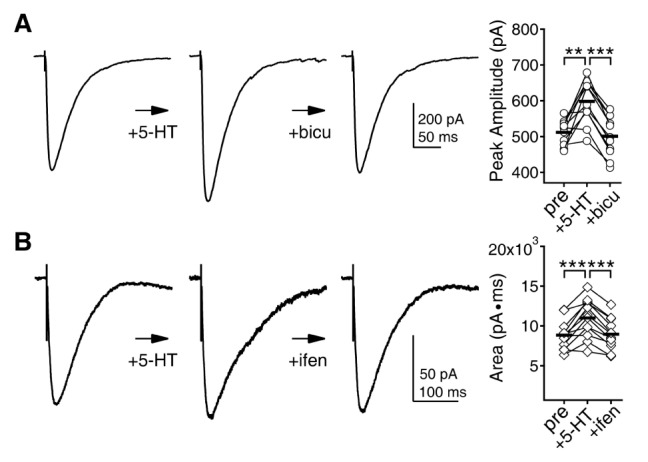
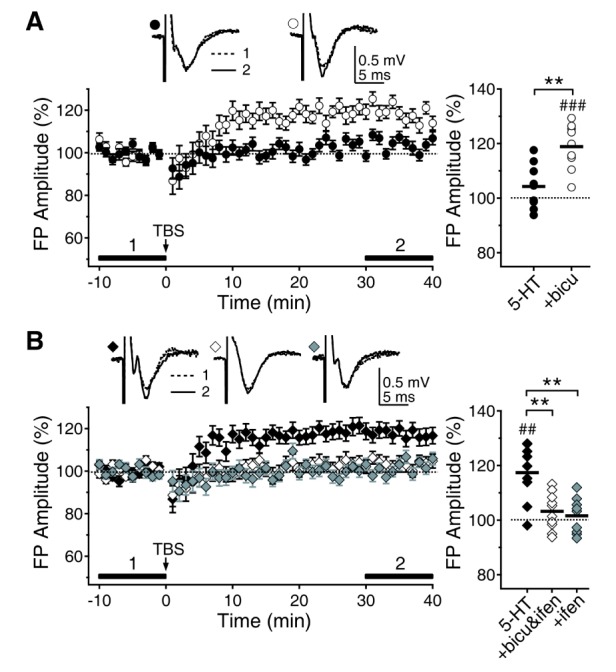
- Serotonin [5-hydroxytryptamine (5-HT)] regulates synaptic plasticity in the visual cortex. Although the effects of 5-HT on plasticity showed huge diversity depending on the ages of animals and species, it has been unclear how 5-HT can show such diverse effects. In the rat visual cortex, 5-HT suppressed long-term potentiation (LTP) at 5 weeks but enhanced LTP at 8 weeks. We speculated that this difference may originate from differential regulation of neurotransmission by 5-HT between the age groups. Thus, we investigated the effects of 5-HT on apha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPAR)-, gamma-aminobutyric acid receptor type A (GABA(A)R)-, and N-methyl-D-aspartic acid receptor (NMDAR)-mediated neurotransmissions and their involvement in the differential regulation of plasticity between 5 and 8 weeks. AMPAR-mediated currents were not affected by 5-HT at both 5 and 8 weeks. GABA(A)R-mediated currents were enhanced by 5-HT at both age groups. However, 5-HT enhanced NMDAR-mediated currents only at 8 weeks. The enhancement of NMDAR-mediated currents appeared to be mediated by the enhanced function of GluN2B subunit-containing NMDAR. The enhanced GABA(A)R- and NMDAR-mediated neurotransmissions were responsible for the suppression of LTP at 5 weeks and the facilitation of LTP at 8 weeks, respectively. These results indicate that the effects of 5-HT on neurotransmission change with development, and the changes may underlie the differential regulation of synaptic plasticity between different age groups. Thus, the developmental changes in 5-HT function should be carefully considered while investigating the 5-HT-mediated metaplastic control of the cortical network.
Keyword
MeSH Terms
Figure
Reference
-
1. Levelt CN, Hübener M. Critical-period plasticity in the visual cortex. Annu Rev Neurosci. 2012; 35:309–330. PMID: 22462544.
Article2. Dudek SM, Friedlander MJ. Developmental down-regulation of LTD in cortical layer IV and its independence of modulation by inhibition. Neuron. 1996; 16:1097–1106. PMID: 8663986.
Article3. Kato N, Artola A, Singer W. Developmental changes in the susceptibility to long-term potentiation of neurones in rat visual cortex slices. Brain Res Dev Brain Res. 1991; 60:43–50. PMID: 1680581.
Article4. Kirkwood A, Lee HK, Bear MF. Co-regulation of long-term potentiation and experience-dependent synaptic plasticity in visual cortex by age and experience. Nature. 1995; 375:328–331. PMID: 7753198.
Article5. Sale A, Berardi N, Spolidoro M, Baroncelli L, Maffei L. GABAergic inhibition in visual cortical plasticity. Front Cell Neurosci. 2010; 4:10. PMID: 20407586.
Article6. Jiang B, Huang ZJ, Morales B, Kirkwood A. Maturation of GABAergic transmission and the timing of plasticity in visual cortex. Brain Res Brain Res Rev. 2005; 50:126–133. PMID: 16024085.
Article7. Erisir A, Harris JL. Decline of the critical period of visual plasticity is concurrent with the reduction of NR2B subunit of the synaptic NMDA receptor in layer 4. J Neurosci. 2003; 23:5208–5218. PMID: 12832545.
Article8. Berardi N, Pizzorusso T, Maffei L. Extracellular matrix and visual cortical plasticity: freeing the synapse. Neuron. 2004; 44:905–908. PMID: 15603733.9. He HY, Hodos W, Quinlan EM. Visual deprivation reactivates rapid ocular dominance plasticity in adult visual cortex. J Neurosci. 2006; 26:2951–2955. PMID: 16540572.
Article10. Maya-Vetencourt JF, Baroncelli L, Viegi A, Tiraboschi E, Castren E, Cattaneo A, Maffei L. IGF-1 restores visual cortex plasticity in adult life by reducing local GABA levels. Neural Plast. 2012; 2012:250421. PMID: 22720172.
Article11. de Vivo L, Landi S, Panniello M, Baroncelli L, Chierzi S, Mariotti L, Spolidoro M, Pizzorusso T, Maffei L, Ratto GM. Extracellular matrix inhibits structural and functional plasticity of dendritic spines in the adult visual cortex. Nat Commun. 2013; 4:1484. PMID: 23403561.
Article12. Castrén E, Elgersma Y, Maffei L, Hagerman R. Treatment of neurodevelopmental disorders in adulthood. J Neurosci. 2012; 32:14074–14079. PMID: 23055475.13. Sodhi MS, Sanders-Bush E. Serotonin and brain development. Int Rev Neurobiol. 2004; 59:111–174. PMID: 15006487.
Article14. Jang HJ, Cho KH, Park SW, Kim MJ, Yoon SH, Rhie DJ. Effects of serotonin on the induction of long-term depression in the rat visual cortex. Korean J Physiol Pharmacol. 2010; 14:337–343. PMID: 21165334.
Article15. Fink KB, Göthert M. 5-HT receptor regulation of neurotransmitter release. Pharmacol Rev. 2007; 59:360–417. PMID: 18160701.
Article16. Moreau AW, Amar M, Callebert J, Fossier P. Serotonergic modulation of LTP at excitatory and inhibitory synapses in the developing rat visual cortex. Neuroscience. 2013; 238:148–158. PMID: 23454367.
Article17. Gu Q, Singer W. Involvement of serotonin in developmental plasticity of kitten visual cortex. Eur J Neurosci. 1995; 7:1146–1153. PMID: 7582087.
Article18. Edagawa Y, Saito H, Abe K. Endogenous serotonin contributes to a developmental decrease in long-term potentiation in the rat visual cortex. J Neurosci. 2001; 21:1532–1537. PMID: 11222643.
Article19. Maya Vetencourt JF, Sale A, Viegi A, Baroncelli L, De Pasquale R, O'Leary OF, Castrén E, Maffei L. The antidepressant fluoxetine restores plasticity in the adult visual cortex. Science. 2008; 320:385–388. PMID: 18420937.20. Baroncelli L, Sale A, Viegi A, Maya Vetencourt JF, De Pasquale R, Baldini S, Maffei L. Experience-dependent reactivation of ocular dominance plasticity in the adult visual cortex. Exp Neurol. 2010; 226:100–109. PMID: 20713044.
Article21. Park SW, Jang HJ, Cho KH, Kim MJ, Yoon SH, Rhie DJ. Developmental switch of the serotonergic role in the induction of synaptic long-term potentiation in the rat visual cortex. Korean J Physiol Pharmacol. 2012; 16:65–70. PMID: 22416222.
Article22. Lee C, Joo K, Kim MJ, Rhie DJ, Jang HJ. GluN2B-containing N-methyl-D-aspartate receptors compensate for the inhibitory control of synaptic plasticity during the early critical period in the rat visual cortex. J Neurosci Res. 2015; 93:1405–1412. PMID: 26013955.
Article23. Lopez de Armentia M, Sah P. Development and subunit composition of synaptic NMDA receptors in the amygdala: NR2B synapses in the adult central amygdala. J Neurosci. 2003; 23:6876–6883. PMID: 12890782.
Article24. Stocca G, Vicini S. Increased contribution of NR2A subunit to synaptic NMDA receptors in developing rat cortical neurons. J Physiol. 1998; 507:13–24. PMID: 9490809.
Article25. Paoletti P. Molecular basis of NMDA receptor functional diversity. Eur J Neurosci. 2011; 33:1351–1365. PMID: 21395862.
Article26. Joo K, Yoon SH, Rhie DJ, Jang HJ. Phasic and tonic inhibition are maintained respectively by CaMKII and PKA in the rat visual cortex. Korean J Physiol Pharmacol. 2014; 18:517–524. PMID: 25598667.
Article27. Jang HJ, Cho KH, Park SW, Kim MJ, Yoon SH, Rhie DJ. Layer-specific serotonergic facilitation of IPSC in layer 2/3 pyramidal neurons of the visual cortex. J Neurophysiol. 2012; 107:407–416. PMID: 22013240.
Article28. Moreau AW, Amar M, Le Roux N, Morel N, Fossier P. Serotoninergic fine-tuning of the excitation-inhibition balance in rat visual cortical networks. Cereb Cortex. 2010; 20:456–467. PMID: 19520765.29. Pugliese AM, Passani MB, Corradetti R. Effect of the selective 5-HT1A receptor antagonist WAY 100635 on the inhibition of e.p.s.ps produced by 5-HT in the CA1 region of rat hippocampal slices. Br J Pharmacol. 1998; 124:93–100. PMID: 9630348.30. Xu TL, Pang ZP, Li JS, Akaike N. 5-HT potentiation of the GABA(A) response in the rat sacral dorsal commissural neurones. Br J Pharmacol. 1998; 124:779–787. PMID: 9690871.
Article31. Feng J, Cai X, Zhao J, Yan Z. Serotonin receptors modulate GABA(A) receptor channels through activation of anchored protein kinase C in prefrontal cortical neurons. J Neurosci. 2001; 21:6502–6511. PMID: 11517239.32. Luhmann HJ, Prince DA. Control of NMDA receptor-mediated activity by GABAergic mechanisms in mature and developing rat neocortex. Brain Res Dev Brain Res. 1990; 54:287–290. PMID: 1975777.
Article33. Kapur A, Lytton WW, Ketchum KL, Haberly LB. Regulation of the NMDA component of EPSPs by different components of postsynaptic GABAergic inhibition: computer simulation analysis in piriform cortex. J Neurophysiol. 1997; 78:2546–2559. PMID: 9356404.
Article34. Edagawa Y, Saito H, Abe K. Stimulation of the 5-HT1A receptor selectively suppresses NMDA receptor-mediated synaptic excitation in the rat visual cortex. Brain Res. 1999; 827:225–228. PMID: 10320714.
Article35. Chen A, Hough CJ, Li H. Serotonin type II receptor activation facilitates synaptic plasticity via N-methyl-D-aspartate-mediated mechanism in the rat basolateral amygdala. Neuroscience. 2003; 119:53–63. PMID: 12763068.
Article36. Yuen EY, Jiang Q, Chen P, Gu Z, Feng J, Yan Z. Serotonin 5-HT1A receptors regulate NMDA receptor channels through a microtubule-dependent mechanism. J Neurosci. 2005; 25:5488–5501. PMID: 15944377.
Article37. Yuen EY, Jiang Q, Chen P, Feng J, Yan Z. Activation of 5-HT2A/C receptors counteracts 5-HT1A regulation of n-methyl-D-aspartate receptor channels in pyramidal neurons of prefrontal cortex. J Biol Chem. 2008; 283:17194–17204. PMID: 18442977.
Article38. Dyck RH, Cynader MS. Autoradiographic localization of serotonin receptor subtypes in cat visual cortex: transient regional, laminar, and columnar distributions during postnatal development. J Neurosci. 1993; 13:4316–4338. PMID: 8410190.
Article39. Hoyer D, Hannon JP, Martin GR. Molecular, pharmacological and functional diversity of 5-HT receptors. Pharmacol Biochem Behav. 2002; 71:533–554. PMID: 11888546.
Article40. Roth BL, Hamblin MW, Ciaranello RD. Developmental regulation of 5-HT2 and 5-HT1c mRNA and receptor levels. Brain Res Dev Brain Res. 1991; 58:51–58. PMID: 2015654.
Article41. Li QH, Nakadate K, Tanaka-Nakadate S, Nakatsuka D, Cui Y, Watanabe Y. Unique expression patterns of 5-HT2A and 5-HT2C receptors in the rat brain during postnatal development: Western blot and immunohistochemical analyses. J Comp Neurol. 2004; 469:128–140. PMID: 14689478.
Article42. Morilak DA, Somogyi P, Lujan-Miras R, Ciaranello RD. Neurons expressing 5-HT2 receptors in the rat brain: neurochemical identification of cell types by immunocytochemistry. Neuropsychopharmacology. 1994; 11:157–166. PMID: 7865097.
Article43. Abraham WC, Bear MF. Metaplasticity: the plasticity of synaptic plasticity. Trends Neurosci. 1996; 19:126–130. PMID: 8658594.
Article44. Wang Y, Gu Q, Cynader MS. Blockade of serotonin-2C receptors by mesulergine reduces ocular dominance plasticity in kitten visual cortex. Exp Brain Res. 1997; 114:321–328. PMID: 9166921.
Article45. Kojic L, Gu Q, Douglas RM, Cynader MS. Serotonin facilitates synaptic plasticity in kitten visual cortex: an in vitro study. Brain Res Dev Brain Res. 1997; 101:299–304. PMID: 9263606.
Article46. Edagawa Y, Saito H, Abe K. Serotonin inhibits the induction of long-term potentiation in rat primary visual cortex. Prog Neuropsychopharmacol Biol Psychiatry. 1998; 22:983–997. PMID: 9789882.
Article47. Jang HJ, Cho KH, Joo K, Kim MJ, Rhie DJ. Differential modulation of phasic and tonic inhibition underlies serotonergic suppression of long-term potentiation in the rat visual cortex. Neuroscience. 2015; 301:351–362. PMID: 26086544.
Article48. Hulme SR, Jones OD, Raymond CR, Sah P, Abraham WC. Mechanisms of heterosynaptic metaplasticity. Philos Trans R Soc Lond B Biol Sci. 2013; 369:20130148. PMID: 24298150.
Article49. Chevaleyre V, Castillo PE. Endocannabinoid-mediated metaplasticity in the hippocampus. Neuron. 2004; 43:871–881. PMID: 15363397.
Article50. Yashiro K, Corlew R, Philpot BD. Visual deprivation modifies both presynaptic glutamate release and the composition of perisynaptic/extrasynaptic NMDA receptors in adult visual cortex. J Neurosci. 2005; 25:11684–11692. PMID: 16354927.
Article51. Philpot BD, Sekhar AK, Shouval HZ, Bear MF. Visual experience and deprivation bidirectionally modify the composition and function of NMDA receptors in visual cortex. Neuron. 2001; 29:157–169. PMID: 11182088.
Article52. Gagolewicz PJ, Dringenberg HC. NR2B-subunit dependent facilitation of long-term potentiation in primary visual cortex following visual discrimination training of adult rats. Eur J Neurosci. 2011; 34:1222–1229. PMID: 21895803.
Article53. Hager AM, Gagolewicz PJ, Rodier S, Kuo MC, Dumont ÉC, Dringenberg HC. Metaplastic up-regulation of LTP in the rat visual cortex by monocular visual training: requirement of task mastery, hemispheric specificity, and NMDA-GluN2B involvement. Neuroscience. 2015; 293:171–186. PMID: 25711939.
Article54. Park JM, Jung SC, Eun SY. Long-term synaptic plasticity: circuit perturbation and stabilization. Korean J Physiol Pharmacol. 2014; 18:457–460. PMID: 25598658.
Article55. Hensch TK. Critical period plasticity in local cortical circuits. Nat Rev Neurosci. 2005; 6:877–888. PMID: 16261181.
Article56. Hensch TK. Controlling the critical period. Neurosci Res. 2003; 47:17–22. PMID: 12941442.
Article57. Griffen TC, Maffei A. GABAergic synapses: their plasticity and role in sensory cortex. Front Cell Neurosci. 2014; 8:91. PMID: 24723851.58. Maffei A. Enriching the environment to disinhibit the brain and improve cognition. Front Cell Neurosci. 2012; 6:53. PMID: 23162430.
Article59. Harauzov A, Spolidoro M, DiCristo G, De Pasquale R, Cancedda L, Pizzorusso T, Viegi A, Berardi N, Maffei L. Reducing intracortical inhibition in the adult visual cortex promotes ocular dominance plasticity. J Neurosci. 2010; 30:361–371. PMID: 20053917.
Article60. Gu Q. Contribution of acetylcholine to visual cortex plasticity. Neurobiol Learn Mem. 2003; 80:291–301. PMID: 14521871.
Article61. Kirkwood A, Rozas C, Kirkwood J, Perez F, Bear MF. Modulation of long-term synaptic depression in visual cortex by acetylcholine and norepinephrine. J Neurosci. 1999; 19:1599–1609. PMID: 10024347.
Article62. Seol GH, Ziburkus J, Huang S, Song L, Kim IT, Takamiya K, Huganir RL, Lee HK, Kirkwood A. Neuromodulators control the polarity of spike-timing-dependent synaptic plasticity. Neuron. 2007; 55:919–929. PMID: 17880895.
Article63. Maya Vetencourt JF, Tiraboschi E, Spolidoro M, Castrén E, Maffei L. Serotonin triggers a transient epigenetic mechanism that reinstates adult visual cortex plasticity in rats. Eur J Neurosci. 2011; 33:49–57. PMID: 21156002.64. Celesia GG. Visual plasticity and its clinical applications. J Physiol Anthropol Appl Human Sci. 2005; 24:23–27.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Role of NMDA Receptor in Learning and Memory
- Developmental Switch of the Serotonergic Role in the Induction of Synaptic Long-term Potentiation in the Rat Visual Cortex
- Effect of Fluoxetine on the Induction of Long-term Potentiation in Rat Frontal Cortex
- The Study on the Role of NMDA Receptors in Rat Visual Cortex
- A study on the role of N-methyl-D-aspartate receptors in the rat visual cortex