J Vet Sci.
2014 Dec;15(4):519-528. 10.4142/jvs.2014.15.4.519.
Enhancing effects of serum-rich and cytokine-supplemented culture conditions on developing blastocysts and deriving porcine parthenogenetic embryonic stem cells
- Affiliations
-
- 1Animal and Plant Quarantine Agency, Anyang 430-757, Korea. virusmania@korea.kr
- 2College of Veterinary Medicine, Kangwon National University, Chuncheon 200-701, Korea.
- 3College of Agriculture and Life Sciences, Seoul National University, Seoul 151-742, Korea.
- 4ATGen Ltd., Seongnam 463-400, Korea.
- KMID: 2070238
- DOI: http://doi.org/10.4142/jvs.2014.15.4.519
Abstract
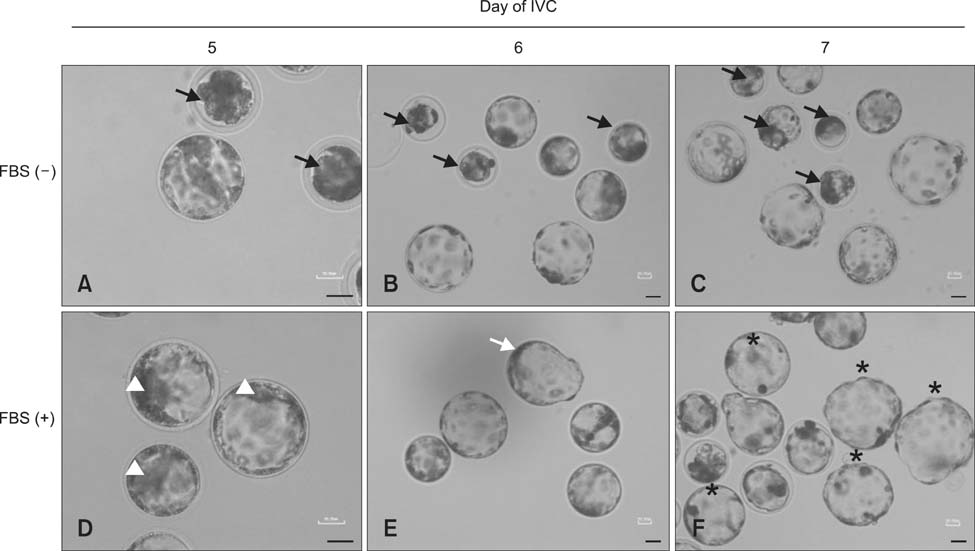
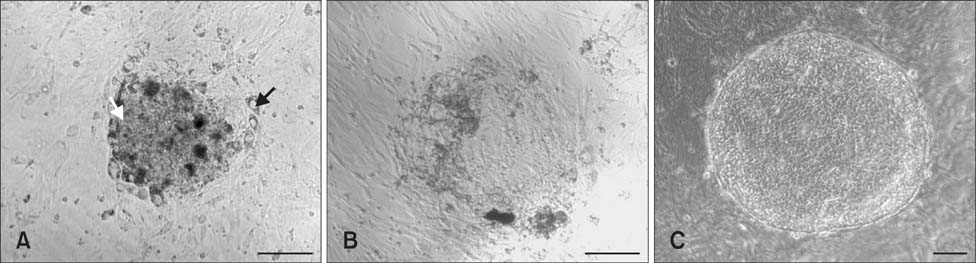
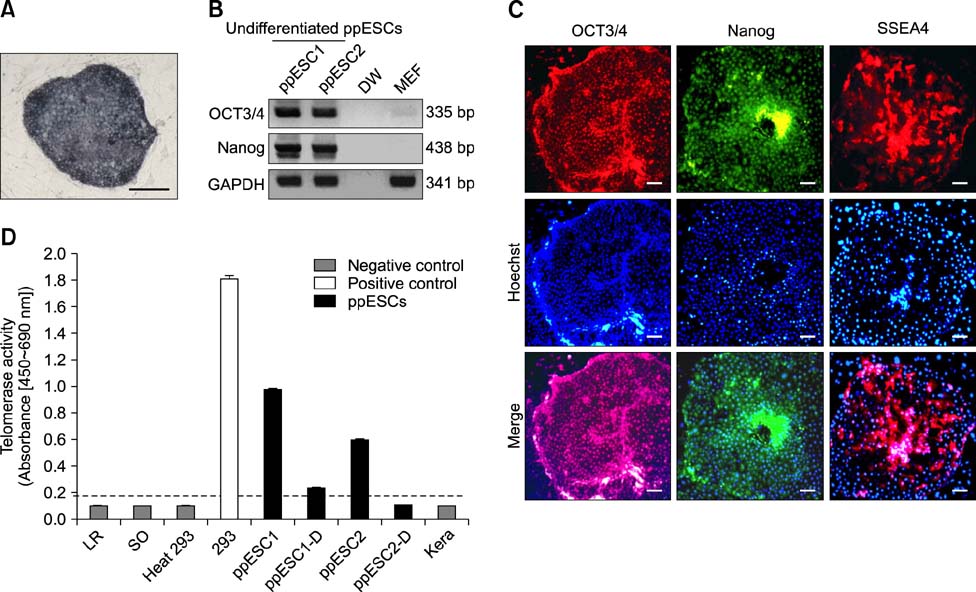
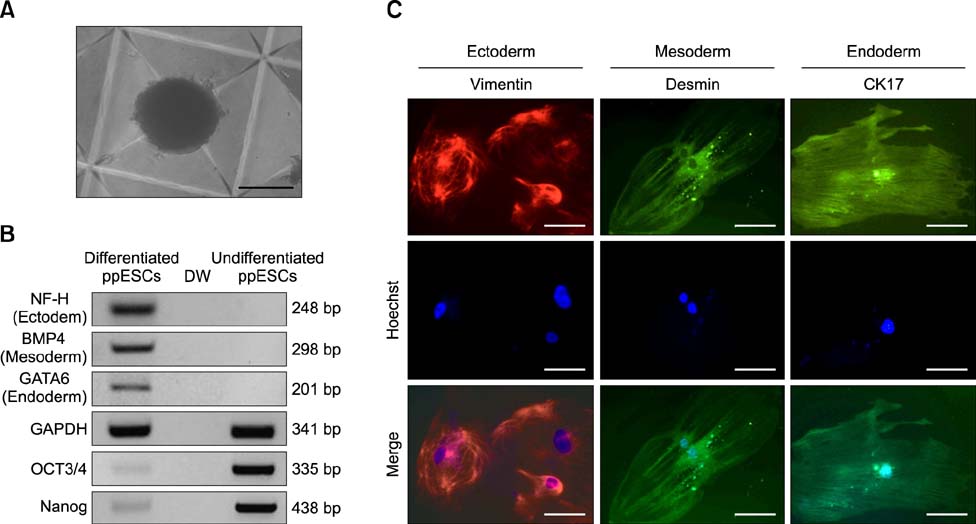
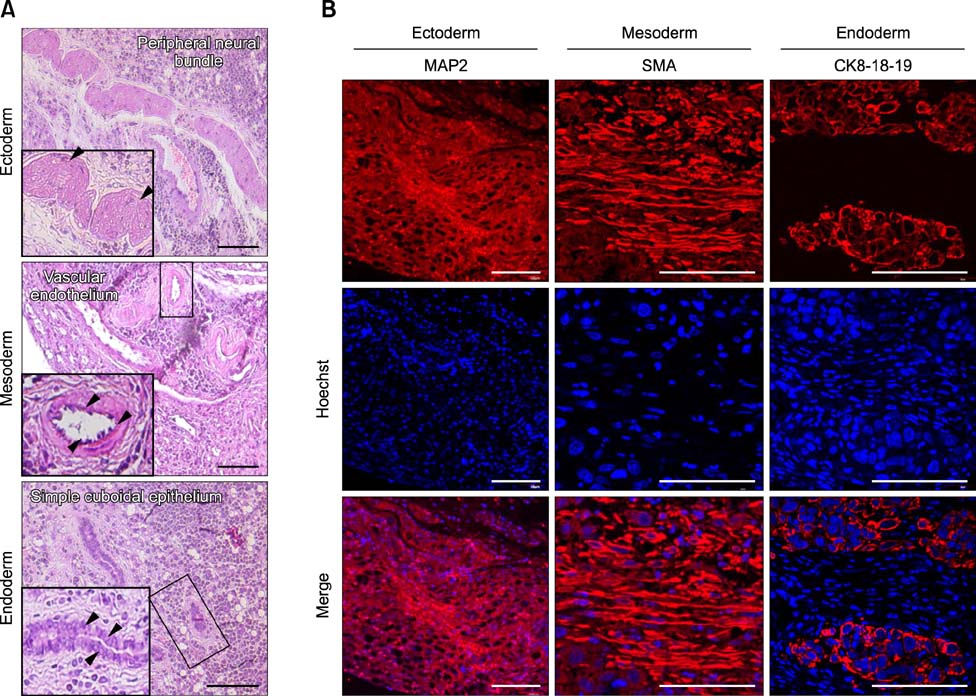
- The present study was conducted to develop an effective method for establishment of porcine parthenogenetic embryonic stem cells (ppESCs) from parthenogenetically activated oocyte-derived blastocysts. The addition of 10% fetal bovine serum (FBS) to the medium on the 3rd day of oocyte culturing improved the development of blastocysts, attachment of inner cell masses (ICMs) onto feeder cells, and formation of primitive ppESC colonies. ICM attachment was further enhanced by basic fibroblast growth factor, stem cell factor, and leukemia inhibitory factor. From these attached ICMs, seven ppESC lines were established. ppESC pluripotency was verified by strong enzymatic alkaline phosphatase activity and the expression of pluripotent markers OCT3/4, Nanog, and SSEA4. Moreover, the ppESCs were induced to form an embryoid body and teratoma. Differentiation into three germ layers (ectoderm, mesoderm, and endoderm) was confirmed by the expression of specific markers for the layers and histological analysis. In conclusion, data from the present study suggested that our modified culture conditions using FBS and cytokines are highly useful for improving the generation of pluripotent ppESCs.
MeSH Terms
Figure
Reference
-
1. Bendall SC, Stewart MH, Menendez P, George D, Vijayaragavan K, Werbowetski-Ogilvie T, Ramos-Mejia V, Rouleau A, Yang J, Bossé M, Lajoie G, Bhatia M. IGF and FGF cooperatively establish the regulatory stem cell niche of pluripotent human cells in vitro. Nature. 2007; 448:1015–1021.
Article2. Brevini TAL, Antonini S, Cillo F, Crestan M, Gandolfi F. Porcine embryonic stem cells: facts, challenges and hopes. Theriogenology. 2007; 68:Suppl 1. S206–S213.
Article3. Brevini TAL, Pennarossa G, Attanasio L, Vanelli A, Gasparrini B, Gandolfi F. Culture conditions and signalling networks promoting the establishment of cell lines from parthenogenetic and biparental pig embryos. Stem Cell Rev. 2010; 6:484–495.
Article4. Brevini TAL, Vassena R, Francisci C, Gandolfi F. Role of adenosine triphosphate, active mitochondria, and microtubules in the acquisition of developmental competence of parthenogenetically activated pig oocytes. Biol Reprod. 2005; 72:1218–1223.
Article5. Campbell JM, Lane M, Vassiliev I, Nottle MB. Use of insulin to increase epiblast cell number: towards a new approach for improving ESC isolation from human embryos. Biomed Res Int. 2013; 2013:150901.
Article6. Dobrinsky JR, Johnson LA, Rath D. Development of a culture medium (BECM-3) for porcine embryos: effects of bovine serum albumin and fetal bovine serum on embryo development. Biol Reprod. 1996; 55:1069–1074.
Article7. Esteban MA, Peng M, Deli Z, Cai J, Yang J, Xu J, Lai L, Pei D. Porcine induced pluripotent stem cells may bridge the gap between mouse and human iPS. IUBMB Life. 2010; 62:277–282.
Article8. Ezashi T, Telugu BPVL, Alexenko AP, Sachdev S, Sinha S, Roberts RM. Derivation of induced pluripotent stem cells from pig somatic cells. Proc Natl Acad Sci U S A. 2009; 106:10993–10998.
Article9. Fangerau H. Can artificial parthenogenesis sidestep ethical pitfalls in human therapeutic cloning? An historical perspective. J Med Ethics. 2005; 31:733–735.
Article10. Glabowski W. The protective effect of stem cell factor (SCF) on in vitro development of preimplantation mouse embryos. Ann Acad Med Stetin. 2005; 51:83–93.11. Glabowski W, Wiszniewska B, Kurzawa R. Protective potential of SCF for mice preimplantation embryos cultured in vitro in suboptimal conditions. J Assist Reprod Genet. 2008; 25:395–402.
Article12. Han MS, Niwa K. Effects of BSA and fetal bovine serum in culture medium on development of rat embryos. J Reprod Dev. 2003; 49:235–242.
Article13. Hardy K, Spanos S. Growth factor expression and function in the human and mouse preimplantation embryo. J Endocrinol. 2002; 172:221–236.
Article14. Harvey MB, Kaye PL. Insulin increases the cell number of the inner cell mass and stimulates morphological development of mouse blastocysts in vitro. Development. 1990; 110:963–967.
Article15. Huppertz B, Herrler A. Regulation of proliferation and apoptosis during development of the preimplantation embryo and the placenta. Birth Defects Res C Embryo Today. 2005; 75:249–261.
Article16. Jin M, Wu A, Dorzhin S, Yue Q, Ma Y, Liu D. Culture conditions for bovine embryonic stem cell-like cells isolated from blastocysts after external fertilization. Cytotechnology. 2012; 64:379–389.
Article17. Karja NW, Otoi T, Murakami M, Yuge M, Fahrudin M, Suzuki T. Effect of protein supplementation on development to the hatching and hatched blastocyst stages of cat IVF embryos. Reprod Fertil Dev. 2002; 14:291–296.
Article18. Kim HS, Son HY, Kim S, Lee GS, Park CH, Kang SK, Lee BC, Hwang WS, Lee CK. Isolation and initial culture of porcine inner cell masses derived from in vitro-produced blastocysts. Zygote. 2007; 15:55–63.
Article19. Liu K, Ji G, Mao J, Liu M, Wang L, Chen C, Liu L. Generation of porcine-induced pluripotent stem cells by using OCT4 and KLF4 porcine factors. Cell Reprogram. 2012; 14:505–513.
Article20. Ma SF, Liu XY, Miao DQ, Han ZB, Zhang X, Miao YL, Yanagimachi R, Tan JH. Parthenogenetic activation of mouse oocytes by strontium chloride: a search for the best conditions. Theriogenology. 2005; 64:1142–1157.
Article21. Michalska AE. Isolation and propagation of mouse embryonic fibroblasts and preparation of mouse embryonic feeder layer cells. Curr Protoc Stem Cell Biol. 2007; Suppl 3. Unit1C.3.1–Unit1C.3.17.22. Miyoshi K, Taguchi Y, Sendai Y, Hoshi H, Sato E. Establishment of a porcine cell line from in vitro-produced blastocysts and transfer of the cells into enucleated oocytes. Biol Reprod. 2000; 62:1640–1646.
Article23. Nichols J, Smith A. The origin and identity of embryonic stem cells. Development. 2011; 138:3–8.
Article24. Park JK, Kim HS, Uh KJ, Choi KH, Kim HM, Lee T, Yang BC, Kim HJ, Ka HH, Kim H, Lee CK. Primed pluripotent cell lines derived from various embryonic origins and somatic cells in pig. PLoS One. 2013; 8:e52481.
Article25. Piedrahita JA, Anderson GB, BonDurant RH. On the isolation of embryonic stem cells: comparative behavior of murine, porcine and ovine embryos. Theriogenology. 1990; 34:879–901.
Article26. Price PJ, Gregory EA. Relationship between in vitro growth promotion and biophysical and biochemical properties of the serum supplement. In Vitro. 1982; 18:576–584.
Article27. Prokhorova TA, Harkness LM, Frandsen U, Ditzel N, Schrøder HD, Burns JS, Kassem M. Teratoma formation by human embryonic stem cells is site dependent and enhanced by the presence of Matrigel. Stem Cells Dev. 2009; 18:47–54.
Article28. Revazova ES, Turovets NA, Kochetkova OD, Kindarova LB, Kuzmichev LN, Janus JD, Pryzhkova MV. Patient-specific stem cell lines derived from human parthenogenetic blastocysts. Cloning Stem Cells. 2007; 9:432–449.
Article29. Shirazi A, Bahiraee A, Ahmadi E, Nazari H, Heidari B, Borjian S. The effect of the duration of in vitro maturation (IVM) on parthenogenetic development of ovine oocytes. Avicenna J Med Biotechnol. 2009; 1:181–191.30. Shiue YL, Liou JF, Shiau JW, Yang JR, Chen YH, Tailiu JJ, Chen LR. In vitro culture period but not the passage number influences the capacity of chimera production of inner cell mass and its deriving cells from porcine embryos. Anim Reprod Sci. 2006; 93:134–143.
Article31. Son J, Malaweera DB, Lee E, Shin S, Cho J. Development of in vitro produced porcine embryos according to serum types as macromolecule. J Vet Sci. 2013; 14:315–321.
Article32. Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998; 282:1145–1147.
Article33. Vassiliev I, Vassilieva S, Beebe LFS, Harrison SJ, McIlfatrick SM, Nottle MB. In vitro and in vivo characterization of putative porcine embryonic stem cells. Cell Reprogram. 2010; 12:223–230.
Article34. Zhang K, Wei HX, Zhang YH, Wang SH, Li Y, Dai YP, Li N. Effects of ghrelin on in vitro development of porcine in vitro fertilized and parthenogenetic embryos. J Reprod Dev. 2007; 53:647–653.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Parthenogenetic Mouse Embryonic Stem Cells have Similar Characteristics to In Vitro Fertilization mES Cells
- Developmental competence of chimeric porcine embryos through the aggregation of parthenogenetic embryos and somatic cell nuclear transfer embryos
- Research Progress and Future Perspectives in Animal Stem Cell Research
- Hematopoietic Stem Cells Culture, Expansion and Differentiation: An Insight into Variable and Available Media
- Isolation and culture of porcine primary fetal progenitors and neurons from the developing dorsal telencephalon