J Vet Sci.
2014 Dec;15(4):485-493. 10.4142/jvs.2014.15.4.485.
N-acetylcysteine protects against cadmium-induced oxidative stress in rat hepatocytes
- Affiliations
-
- 1College of Veterinary Medicine, Yangzhou University, Yangzhou 225009, China. liuzongping@yzu.edu.cn
- 2College of Animal Science and Technology, Henan University of Science and Technology, Luoyang 471003, China.
- KMID: 2070234
- DOI: http://doi.org/10.4142/jvs.2014.15.4.485
Abstract
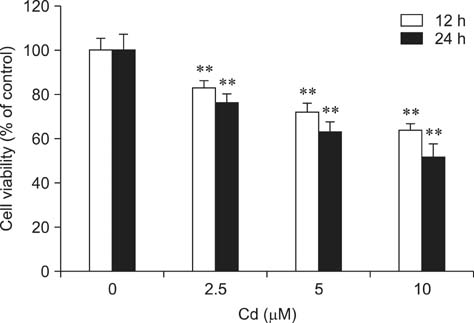
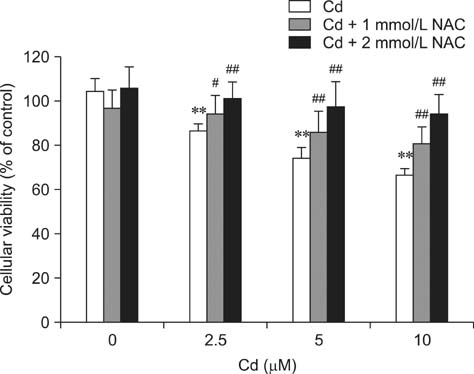
- Cadmium (Cd) is a well-known hepatotoxic environmental pollutant. We used rat hepatocytes as a model to study oxidative damage induced by Cd, effects on the antioxidant systems, and the role of N-acetylcysteine (NAC) in protecting cells against Cd toxicity. Hepatocytes were incubated for 12 and 24 h with Cd (2.5, 5, 10 microM). Results showed that Cd can induce cytotoxicity: 10 microM resulted in 36.2% mortality after 12 h and 47.8% after 24 h. Lactate dehydrogenase, aspartate aminotransferase, and alanine aminotransferase activities increased. Additionally, reactive oxygen species (ROS) generation increased in Cd-treated hepatocytes along with malondialdehyde levels. Glutathione concentrations significantly decreased after treatment with Cd for 12 h but increased after 24 h of Cd exposure. In contrast, glutathione peroxidase activity significantly increased after treatment with Cd for 12 h but decreased after 24 h. superoxide dismutase and catalase activities increased at 12 h and 24 h. glutathione S-transferase and glutathione reductase activities decreased, but not significantly. Rat hepatocytes incubated with NAC and Cd simultaneously had significantly increased viability and decreased Cd-induced ROS generation. Our results suggested that Cd induces ROS generation that leads to oxidative stress. Moreover, NAC protects rat hepatocytes from cytotoxicity associated with Cd.
Keyword
MeSH Terms
-
Acetylcysteine/*metabolism
Animals
Antioxidants/*metabolism
Cadmium/*toxicity
Cell Survival/drug effects
Cells, Cultured
Environmental Pollutants/*toxicity
Hepatocytes/drug effects/metabolism
*Oxidative Stress
Rats
Rats, Sprague-Dawley
Reactive Oxygen Species/*metabolism
Acetylcysteine
Antioxidants
Cadmium
Environmental Pollutants
Reactive Oxygen Species
Figure
Reference
-
1. Belyaeva EA, Korotkov SM. Mechanism of primary Cd2+-induced rat liver mitochondria dysfunction: discrete modes of Cd2+ action on calcium and thiol-dependent domains. Toxicol Appl Pharmacol. 2003; 192:56–68.
Article2. Bolduc JS, Denizeau F, Jumarie C. Cadmium-induced mitochondrial membrane-potential dissipation does not necessarily require cytosolic oxidative stress: studies using rhodamine-123 fluorescence unquenching. Toxicol Sci. 2004; 77:299–306.
Article3. Bonaventura J, Schroeder WA, Fang S. Human erythrocyte catalase: an improved method of isolation and a reevaluation of reported properties. Arch Biochem Biophys. 1972; 150:606–617.
Article4. Bridges CC, Zalups RK. Molecular and ionic mimicry and the transport of toxic metals. Toxicol Appl Pharmacol. 2005; 204:274–308.
Article5. Chance B, Greenstein DS, Roughton FJW. The mechanism of catalase action. I. Steady state analysis. Arch Biochem Biophys. 1952; 37:301–321.6. Chin TA, Templeton DM. Protective elevations of glutathione and metallothionein in cadmium-exposed mesangial cells. Toxicology. 1993; 77:145–156.
Article7. Dalton TP, Shertzer HG, Puga A. Regulation of gene expression by reactive oxygen. Annu Rev Pharmacol Toxicol. 1999; 39:67–101.
Article8. Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959; 82:70–77.
Article9. Esterbauer H, Cheeseman KH. Determination of aldehydic lipid peroxidation products: malonaldehyde and 4-hydroxynonenal. Methods Enzymol. 1990; 186:407–421.10. Flohé L, Günzler WA. Assays of glutathione peroxidase. Methods Enzymol. 1984; 105:114–121.11. Funakoshi T, Ueda K, Shimada H, Kojima S. Effects of dithiocarbamates on toxicity of cadmium in rat primary hepatocyte cultures. Toxicology. 1997; 116:99–107.
Article12. Gaubin Y, Vaissade F, Croute F, Beau B, Soleilhavoup JP, Murat JC. Implication of free radicals and glutathione in the mechanism of cadmium-induced expression of stress proteins in the A549 human lung cell-line. Biochim Biophys Acta. 2000; 1495:4–13.
Article13. Habig WH, Pabst MJ, Jakoby WB. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem. 1974; 249:7130–7139.14. Hatcher EL, Chen Y, Kang YJ. Cadmium resistance in A549 cells correlates with elevated glutathione content but not antioxidant enzymatic activities. Free Radic Biol Med. 1995; 19:805–812.
Article15. Hinkle PM, Kinsella PA, Osterhoudt KC. Cadmium uptake and toxicity via voltage-sensitive calcium channels. J Biol Chem. 1987; 262:16333–16337.
Article16. Hussain T, Shukla GS, Chandra SV. Effects of cadmium on superoxide dismutase and lipid peroxidation in liver and kidney of growing rats: in vivo and in vitro studies. Pharmacol Toxicol. 1987; 60:355–358.
Article17. Kakkar P, Das B, Viswanathan PN. A modified spectrophotometric assay of superoxide dismutase. Indian J Biochem Biophys. 1984; 21:130–132.18. Ketterer B. Detoxication reactions of glutathione and glutathione transferases. Xenobiotica. 1986; 16:957–973.
Article19. Kreamer BL, Staecker JL, Sawada N, Sattler GL, Hsia MT, Pitot HC. Use of a low-speed, iso-density percoll centrifugation method to increase the viability of isolated rat hepatocyte preparations. In Vitro Cell Dev Biol. 1986; 22:201–211.
Article20. Leelavinothan P, Kalist S. Beneficial effect of hesperetin on cadmium induced oxidative stress in rats: an in vivo and in vitro study. Eur Rev Med Pharmacol Sci. 2011; 15:992–1002.21. Lemarié A, Lagadic-Gossmann D, Morzadec C, Allain N, Fardel O, Vernhet L. Cadmium induces caspase-independent apoptosis in liver Hep3B cells: role for calcium in signaling oxidative stress-related impairment of mitochondria and relocation of endonuclease G and apoptosis-inducing factor. Free Radic Biol Med. 2004; 36:1517–1531.
Article22. Lin WC, Liao YC, Liau MC, Lii CK, Sheen LY. Inhibitory effect of CDA-II, a urinary preparation, on aflatoxin B1-induced oxidative stress and DNA damage in primary cultured rat hepatocytes. Food Chem Toxicol. 2006; 44:546–551.
Article23. Liu T, He W, Yan C, Qi Y, Zhang Y. Roles of reactive oxygen species and mitochondria in cadmium-induced injury of liver cells. Toxicol Ind Health. 2011; 27:249–256.
Article24. López E, Arce C, Oset-Gasque MJ, Cañadas S, González MP. Cadmium induces reactive oxygen species generation and lipid peroxidation in cortical neurons in culture. Free Radic Biol Med. 2006; 40:940–951.
Article25. Méplan C, Mann K, Hainaut P. Cadmium induces conformational modifications of wild-type p53 and suppresses p53 response to DNA damage in cultured cells. J Biol Chem. 1999; 274:31663–31670.
Article26. Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983; 65:55–63.
Article27. Müller L. Consequences of cadmium toxicity in rat hepatocytes: mitochondrial dysfunction and lipid peroxidation. Toxicology. 1986; 40:285–295.
Article28. Murugavel P, Pari L. Effects of diallyl tetrasulfide on cadmium-induced oxidative damage in the liver of rats. Hum Exp Toxicol. 2007; 26:527–534.
Article29. Nemmiche S, Chabane-Sari D, Guiraud P. Role of α-tocopherol in cadmium-induced oxidative stress in Wistar rat's blood, liver and brain. Chem Biol Interact. 2007; 170:221–230.
Article30. Newairy AA, El-Sharaky AS, Badreldeen MM, Eweda SM, Sheweita SA. The hepatoprotective effects of selenium against cadmium toxicity in rats. Toxicology. 2007; 242:23–30.
Article31. Nzengue Y, Steiman R, Garrel C, Lefèbvre E, Guiraud P. Oxidative stress and DNA damage induced by cadmium in the human keratinocyte HaCaT cell line: role of glutathione in the resistance to cadmium. Toxicology. 2008; 243:193–206.
Article32. Odewumi CO, Badisa VLD, Le UT, Latinwo LM, Ikediobi CO, Badisa RB, Darling-Reed SF. Protective effects of N-acetylcysteine against cadmium-induced damage in cultured rat normal liver cells. Int J Mol Med. 2011; 27:243–248.
Article33. Poliandri AHB, Cabilla JP, Velardez MO, Bodo CCA, Duvilanski BH. Cadmium induces apoptosis in anterior pituitary cells that can be reversed by treatment with antioxidants. Toxicol Appl Pharmacol. 2003; 190:17–24.
Article34. Prabu SM, Muthumani M, Shagirtha K. Quercetin potentially attenuates cadmium induced oxidative stress mediated cardiotoxicity and dyslipidemia in rats. Eur Rev Med Pharmacol Sci. 2013; 17:582–595.35. Renugadevi J, Prabu SM. Quercetin protects against oxidative stress-related renal dysfunction by cadmium in rats. Exp Toxicol Pathol. 2010; 62:471–481.
Article36. Shih CM, Ko WC, Wu JS, Wei YH, Wang LF, Chang EE, Lo TY, Cheng HH, Chen CT. Mediating of caspase-independent apoptosis by cadmium through the mitochondria-ROS pathway in MRC-5 fibroblasts. J Cell Biochem. 2004; 91:384–397.
Article37. Sinha M, Manna P, Sil PC. Taurine, a conditionally essential amino acid, ameliorates arsenic-induced cytotoxicity in murine hepatocytes. Toxicol In Vitro. 2007; 21:1419–1428.
Article38. Smith IK, Vierheller TL, Thorne CA. Assay of glutathione reductase in crude tissue homogenates using 5,5'-dithiobis(2-nitrobenzoic acid). Anal Biochem. 1988; 175:408–413.
Article39. Wispriyono B, Matsuoka M, Igisu H, Matsuno K. Protection from cadmium cytotoxicity by N-acetylcysteine in LLC-PK1 cells. J Pharmacol Exp Ther. 1998; 287:344–351.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Resveratrol Protects HepG2 and Chang Liver Cells from Oxidative Stress
- Induction of Angiogenic Cytokines in Cultured RPE by Oxidative Stress
- Light Electron Microscopic Study in Rat Livers Following Cadmium Chloride Administration
- Effect of Oxidative Stress on the Expression of Gelatinases in Cultured RPE
- Hypoxia Activates Toll-like Receptor 4 Signaling in Primary Mouse Hepatocytes Through the Receptor Clustering within Lipid Rafts