J Vet Sci.
2014 Dec;15(4):475-483. 10.4142/jvs.2014.15.4.475.
Stanniocalcin-1 protects bovine intestinal epithelial cells from oxidative stress-induced damage
- Affiliations
-
- 1College of Veterinary Medicine, Huazhong Agricultural University, Wuhan 430070, China. vetwu@qq.com
- 2Hubei Key Laboratory of Animal Embryo and Molecular Breeding, Hubei Academy of Agricultural Science, Wuhan 430064, China.
- 3Biotechnology Institute of Animal and Veterinary Science, Sichan Animal Science Academy, Chengdu, 610066, China.
- KMID: 2070233
- DOI: http://doi.org/10.4142/jvs.2014.15.4.475
Abstract
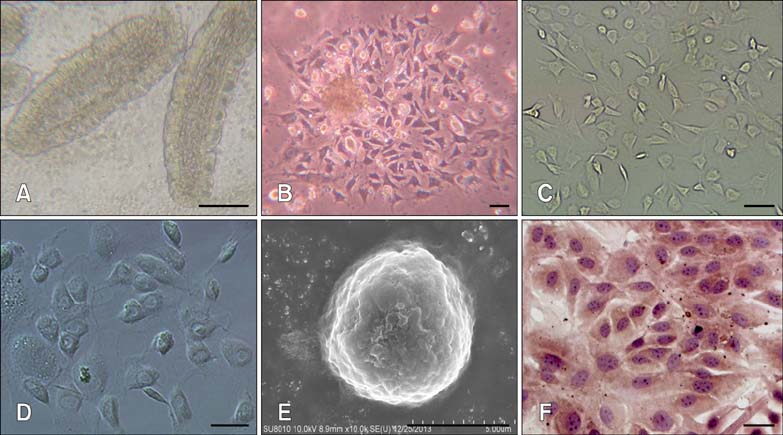
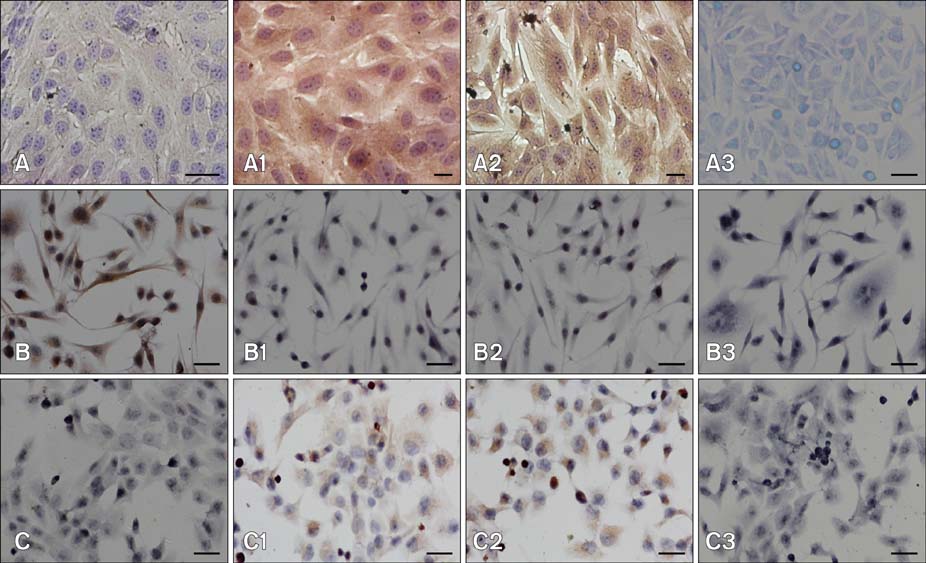
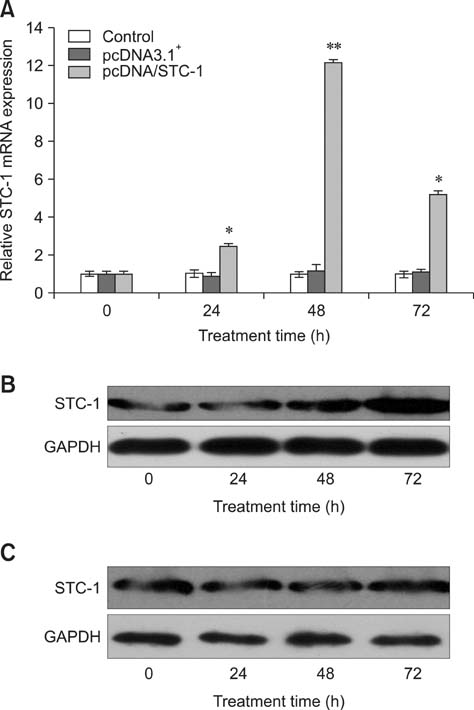
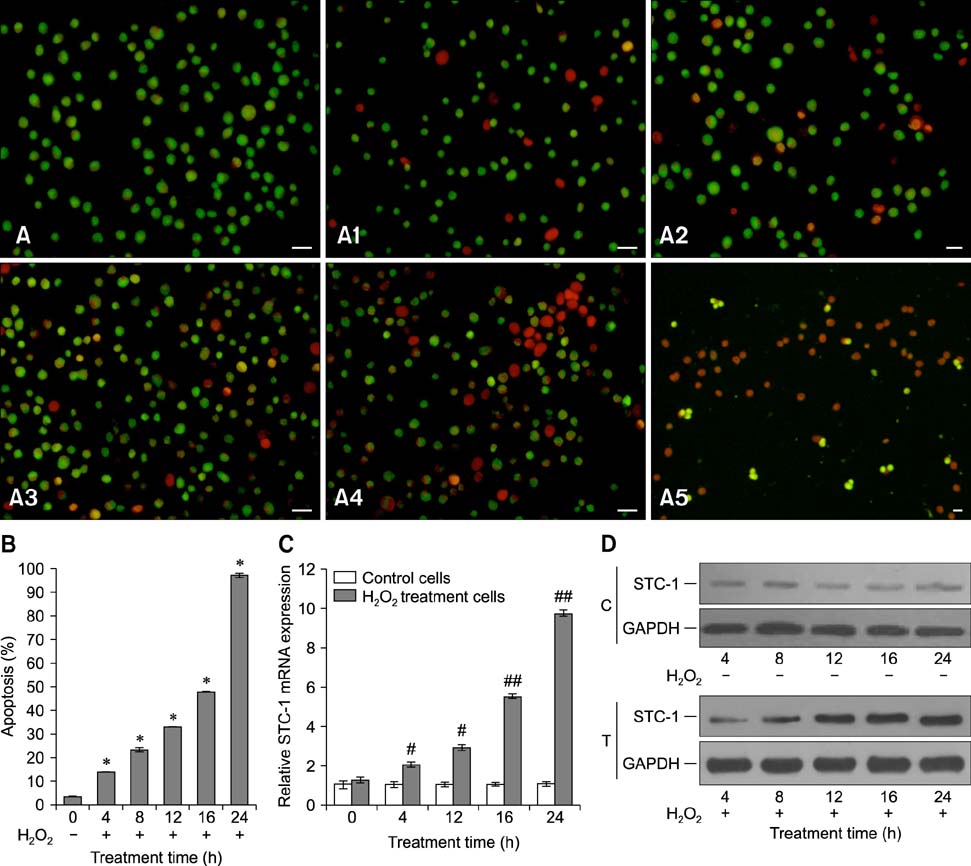
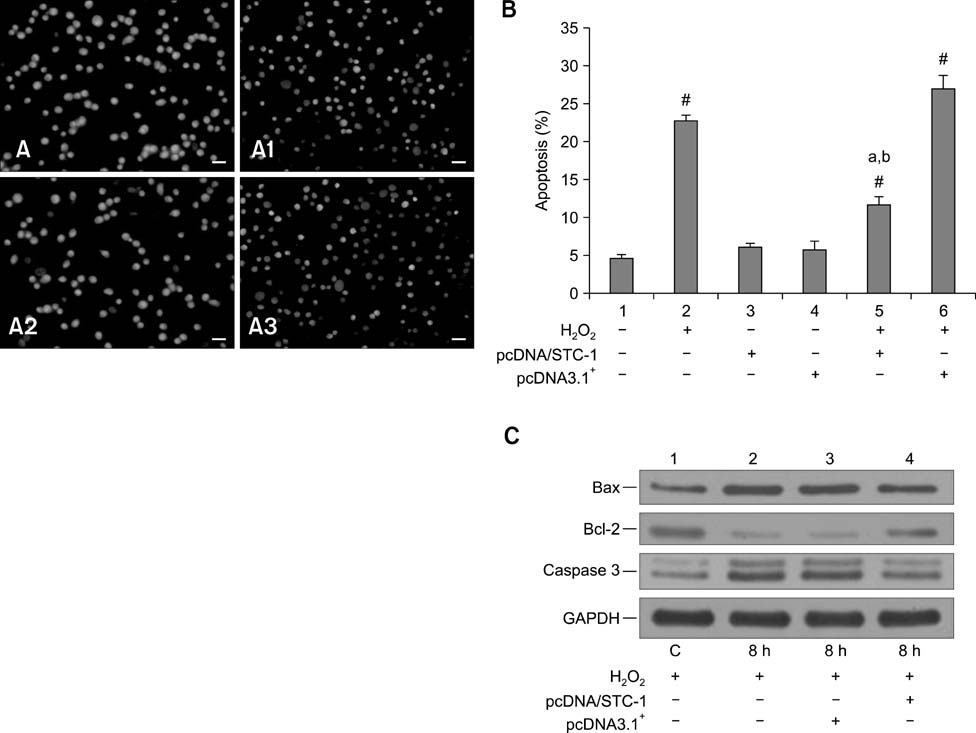
- Chronic enteritis can produce an excess of reactive oxygen species resulting in cellular damage. Stanniocalcin-1(STC-1) reportedly possesses anti-oxidative activity, the aim of this study was to define more clearly the direct contribution of STC-1 to anti-oxidative stress in cattle. In this study, primary intestinal epithelial cells (IECs) were exposed to hydrogen peroxide (H2O2) for different time intervals to mimic chronic enteritis-induced cellular damage. Prior to treatment with 200 microM H2O2, the cells were transfected with a recombinant plasmid for 48 h to over-express STC-1. Acridine orange/ethidium bromide (AO/EB) double staining and trypan blue exclusion assays were then performed to measure cell viability and apoptosis of the cells, respectively. The expression of STC-1 and apoptosis-related proteins in the cells was monitored by real-time PCR and Western blotting. The results indicated that both STC-1 mRNA and protein expression levels positively correlated with the duration of H2O2 treatment. H2O2 damaged the bovine IECs in a time-dependent manner, and this effect was attenuated by STC-1 over-expression. Furthermore, over-expression of STC-1 up-regulated Bcl-2 protein expression and slightly down-regulated caspase-3 production in the damaged cells. Findings from this study suggested that STC-1 plays a protective role in intestinal cells through an antioxidant mechanism.
Keyword
MeSH Terms
-
Animals
Animals, Newborn
Blotting, Western/veterinary
Caspase 3/*genetics/metabolism
Cattle
Cattle Diseases/etiology/*genetics/metabolism
Duodenum/metabolism
Enteritis/etiology/genetics/metabolism/*veterinary
Epithelial Cells/metabolism
*Gene Expression Regulation
Glycoproteins/*genetics/metabolism
Hydrogen Peroxide/pharmacology
Male
Proto-Oncogene Proteins c-bcl-2/*genetics/metabolism
RNA, Messenger/genetics/metabolism
Real-Time Polymerase Chain Reaction/veterinary
Caspase 3
Glycoproteins
Proto-Oncogene Proteins c-bcl-2
RNA, Messenger
Hydrogen Peroxide
Figure
Reference
-
1. Arigami T, Uenosono Y, Ishigami S, Hagihara T, Haraguchi N, Matsushita D, Yanagita S, Nakajo A, Okumura H, Hokita S, Natsugoe S. Expression of stanniocalcin 1 as a potential biomarker of gastric cancer. Oncology. 2012; 83:158–164.
Article2. Bae JY, Ahn SJ, Han W, Noh DY. Peroxiredoxin I and II inhibit H2O2-induced cell death in MCF-7 cell lines. J Cell Biochem. 2007; 101:1038–1045.
Article3. Baioni L, Basini G, Bussolati S, Grasselli F. Stanniocalcin 1 affects redox status of swine granulosa cells. Regul Pept. 2011; 168:45–49.
Article4. Bartsch H, Nair J. Chronic inflammation and oxidative stress in the genesis and perpetuation of cancer: role of lipid peroxidation, DNA damage, and repair. Langenbecks Arch Surg. 2006; 391:499–510.
Article5. Chang ACM, Janosi J, Hulsbeek M, de Jong D, Jeffrey KJ, Noble JR, Reddel RR. A novel human cDNA highly homologous to the fish hormone stanniocalcin. Mol Cell Endocrinol. 1995; 112:241–247.
Article6. De Niu P, Radman DP, Jaworski EM, Deol H, Gentz R, Su J, Olsen HS, Wagner GF. Development of a human stanniocalcin radioimmunoassay: serum and tissue hormone levels and pharmacokinetics in the rat. Mol Cell Endocrinol. 2000; 162:131–144.
Article7. Deol H, Stasko SE, De Niu P, James KA, Wagner GF. Post-natal ontogeny of stanniocalcin gene expression in rodent kidney and regulation by dietary calcium and phosphate. Kidney Int. 2001; 60:2142–2152.
Article8. Föllmann W, Weber S, Birkner S. Primary cell cultures of bovine colon epithelium: isolation and cell culture of colonocytes. Toxicol In Vitro. 2000; 14:435–445.
Article9. Feghali CA, Wright TM. Cytokines in acute and chronic inflammation. Front Biosci. 1997; 2:d12–d26.
Article10. Freshney RI, Freshney MG. Culture of Epithelial Cells. 2nd ed. New York: Wiley-Liss;2004. p. 303–336.11. Huang L, Belousova T, Chen M, DiMattia G, Liu D, Sheikh-Hamad D. Overexpression of stanniocalcin-1 inhibits reactive oxygen species and renal ischemia/reperfusion injury in mice. Kidney Int. 2012; 82:867–877.
Article12. Huang L, Garcia G, Lou Y, Zhou Q, Truong LD, DiMattia G, Lan XR, Lan HY, Wang Y, Sheikh-Hamad D. Anti-inflammatory and renal protective actions of stanniocalcin-1 in a model of anti-glomerular basement membrane glomerulonephritis. Am J Pathol. 2009; 174:1368–1378.
Article13. Kopa Z, Wenzel J, Papp GK, Haidl G. Role of granulocyte elastase and interleukin-6 in the diagnosis of male genital tract inflammation. Andrologia. 2005; 37:188–194.
Article14. La Ragione RM, Cooley WA, Woodward MJ. The role of fimbriae and flagella in the adherence of avian strains of Escherichia coli O78:K80 to tissue culture cells and tracheal and gut explants. J Med Microbiol. 2000; 49:327–338.
Article15. Law AYS, Ching LY, Lai KP, Wong CKC. Identification and characterization of the hypoxia-responsive element in human stanniocalcin-1 gene. Mol Cell Endocrinol. 2010; 314:118–127.
Article16. Lee H, Stabel J, Kehrli ME Jr. Cytokine gene expression in ileal tissues of cattle infected with Mycobacterium paratuberculosis. Vet Immunol Immunopathol. 2001; 82:73–85.
Article17. Liu G, Yang G, Chang B, Mercado-Uribe I, Huang M, Zheng J, Bast RC, Lin SH, Liu J. Stanniocalcin 1 and ovarian tumorigenesis. J Natl Cancer Inst. 2010; 102:812–827.
Article18. Lykkesfeldt J, Svendsen O. Oxidants and antioxidants in disease: oxidative stress in farm animals. Vet J. 2007; 173:502–511.
Article19. McGill G, Shimamura A, Bates RC, Savage RE, Fisher DE. Loss of matrix adhesion triggers rapid transformationselective apoptosis in fibroblasts. J Cell Biol. 1997; 138:901–911.
Article20. Moyer MP, Aust JB. Human colon cells: culture and in vitro transformation. Science. 1984; 224:1445–1447.
Article21. Serlachius M, Andersson LC. Upregulated expression of stanniocalcin-1 during adipogenesis. Exp Cell Res. 2004; 296:256–264.
Article22. Sheikh-Hamad D. Mammalian stanniocalcin-1 activates mitochondrial antioxidant pathways: new paradigms for regulation of macrophages and endothelium. Am J Physiol Renal Physiol. 2010; 298:F248–F254.
Article23. Tremblay G, Delbecchi L, Loiselle MC, Ster C, Wagner GF, Talbot BG, Lacasse P. Serum levels of stanniocalcin-1 in Holstein heifers and cows. Domest Anim Endocrinol. 2009; 36:105–109.
Article24. Wagner GF, Jaworski E. Calcium regulates stanniocalcin mRNA levels in primary cultured rainbow trout corpuscles of stannius. Mol Cell Endocrinol. 1994; 99:315–322.
Article25. Wagner GF, Hampong M, Park CM, Copp DH. Purification, characterization, and bioassay of teleocalcin, a glycoprotein from salmon corpuscles of Stannius. Gen Comp Endocrinol. 1986; 63:481–491.
Article26. Watson PR, Galyov EE, Paulin SM, Jones PW, Wallis TS. Mutation of invH, but Not stn, reduces Salmonella-induced enteritis in cattle. Infect immun. 1998; 66:1432–1438.
Article27. Wiseman H, Halliwell B. Damage to DNA by reactive oxygen and nitrogen species: role in inflammatory disease and progression to cancer. Biochem J. 1996; 313:17–29.
Article28. Wu L, Xi Z, Guo R, Liu S, Yang S, Liu D, Dong S, Guo D. Exogenous ARC down-regulates caspase-3 expression and inhibits apoptosis of broiler chicken cardiomyocytes exposed to hydrogen peroxide. Avian Pathol. 2013; 42:32–37.
Article29. Yeung HY, Lai KP, Chan HY, Mak NK, Wagner GF, Wong CKC. Hypoxia-inducible factor-1-mediated activation of stanniocalcin-1 in human cancer cells. Endocrinology. 2005; 146:4951–4960.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Carbon monoxide releasing molecule-2 protects mice against acute kidney injury through inhibition of ER stress
- Epilepsy and Oxidative Stress
- Galangin (3,5,7-Trihydroxyflavone) Shields Human Keratinocytes from Ultraviolet B-Induced Oxidative Stress
- Overexpressed Mitochondrial Thioredoxin Protects PC12 Cells from Hydrogen Peroxide and Serum-deprivation
- Reduction of TNF alpha-induced oxidative DNA damage product, 8-hydroxy-2'-deoxyguanosine, in L929 cells stably transfected with small heat shock protein