J Vet Sci.
2014 Dec;15(4):465-474. 10.4142/jvs.2014.15.4.465.
Developmental changes in cell proliferation and apoptosis in the normal duck bursa of Fabricius
- Affiliations
-
- 1College of Veterinary Medicine, Sichuan Agricultural University, Ya'an 625014, China. fangjing4109@163.com
- KMID: 2070232
- DOI: http://doi.org/10.4142/jvs.2014.15.4.465
Abstract
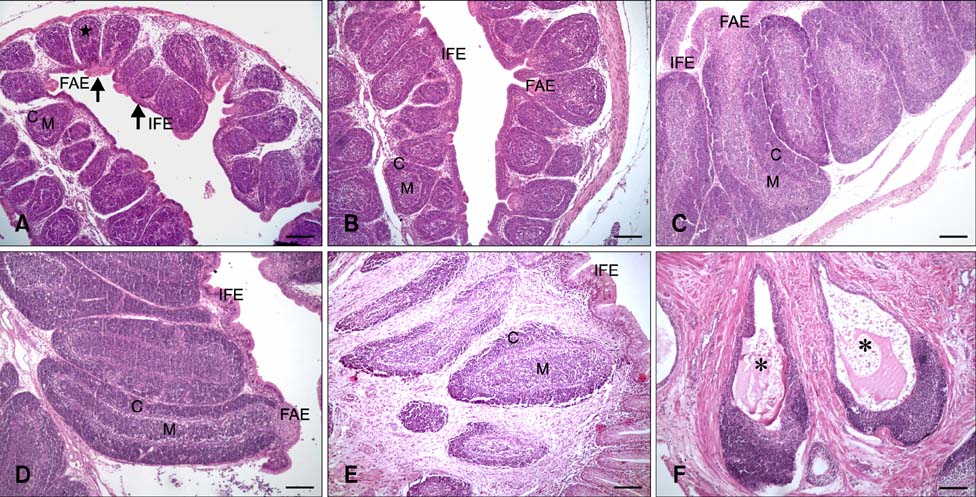
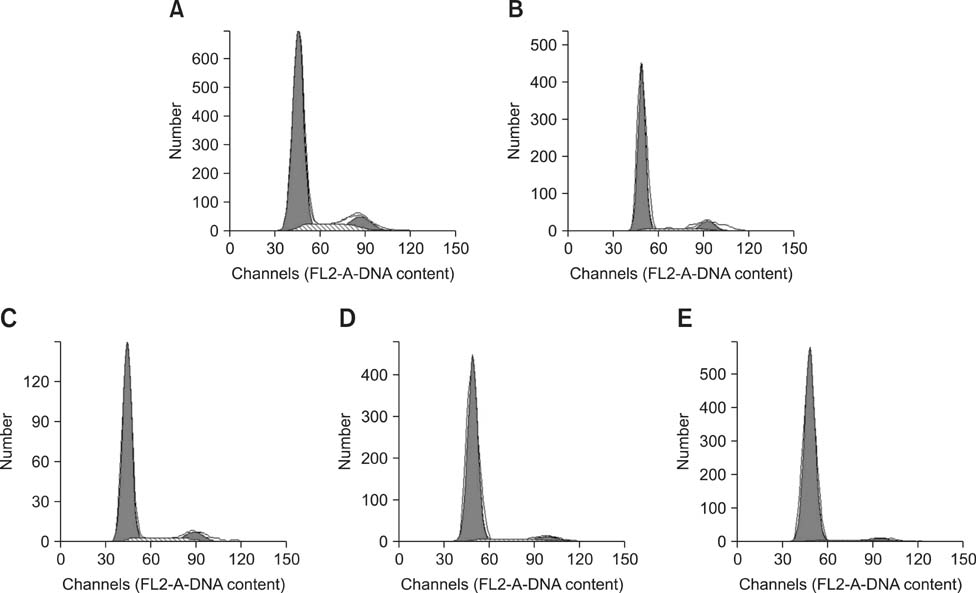
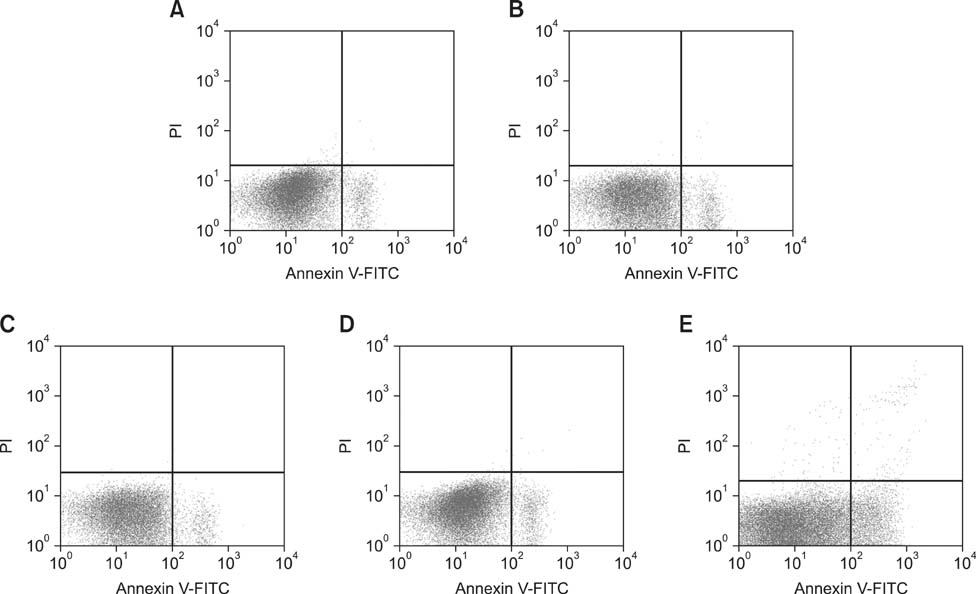
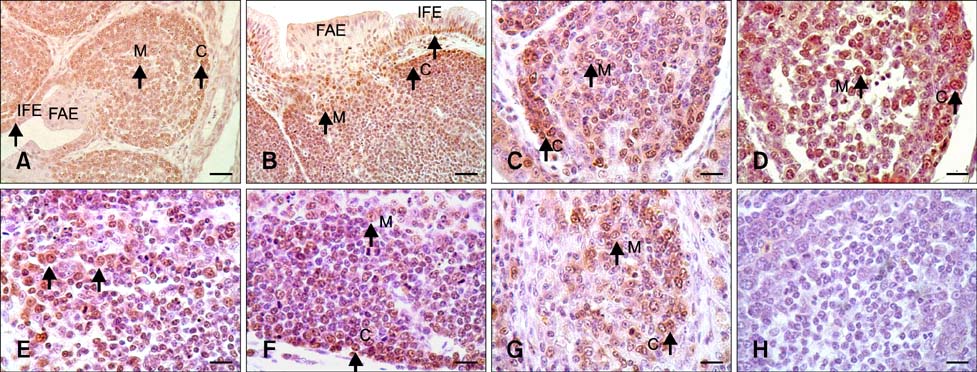
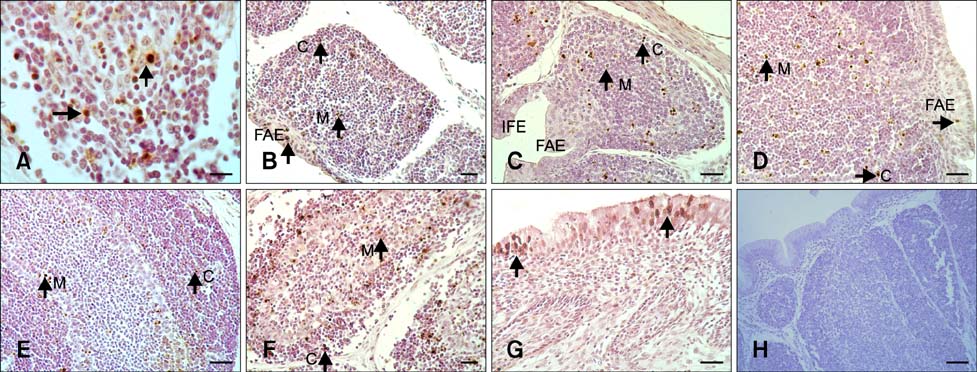
- The aim of this work was to investigate developmental changes in cell proliferation and apoptosis in normal duck bursa of Fabricius using flow cytometry and immunohistochemistry. Studies were carried out on Tianfu ducks on days 24 and 27 of embryogenesis (E24 and E27) along with days 20, 70, and 200 of postnatal development (P20, P70, and P200). Results showed that the percentage of G0/G1 bursa cells significantly increased between E24 and P200 while the percentage of cells in the S phase or G2 + M phase as well as the proliferating index obviously decreased during the same period. Proliferation cell nuclear antigen was detected in lymphocyte and interfollicular epithelium. The proliferative lymphocyte density tended to decrease from E24 to P200. Apoptotic bodies in macrophages, free apoptotic bodies, or nuclei with condensed chromatin in lymphocytes in follicles were identified by transferase-mediated dUTP nick-end labeling. Both flow cytometry and microscopic analysis reveal that the proportion of apoptotic cells and apoptotic lymphocyte density increased from E24 to P20, fell on P70, then rose again on P200. Our foundings demonstrate that cell proliferation decreases and apoptosis increases with age. These changes may account for duck bursa development and involution.
Keyword
MeSH Terms
-
Animals
*Apoptosis
Bursa of Fabricius/*cytology/embryology/growth & development/*physiology
Cell Proliferation
Ducks/embryology/*physiology
Embryo, Nonmammalian/cytology/embryology
Embryonic Development
Epithelium/physiology
Female
Flow Cytometry/veterinary
Immunohistochemistry/veterinary
Lymphocytes/physiology
Male
Figure
Reference
-
1. Butcher GD, Harms RH, Winterfield RW. Relationship between delayed onset of egg production and involution of the bursa of Fabricius in White Leghorn chickens. Avian Dis. 1989; 33:361–364.
Article2. Chen T, Cui Y, Gong T, Bai C, Peng X, Cui H. Inhibition of splenocyte proliferation and spleen growth in young chickens fed high fluoride diets. Fluoride. 2009; 42:203–209.3. Ciriaco E, Píñera PP, Díaz-Esnal B, Laurà R. Age-related changes in the avian primary lymphoid organs (thymus and bursa of Fabricius). Microsc Res Tech. 2003; 62:482–487.
Article4. Fang J, Cui H, Peng X, Chen Z, He M, Tang L. Developmental Changes in cell proliferation and apoptosis in the normal duck thymus. Anat Histol Embryol. 2011; 40:457–465.
Article5. Franchini A, Ottaviani E. Immunoreactive POMC-derived peptides and cytokines in the chicken thymus and bursa of Fabricius microenvironments: age-related changes. J Neuroendocrinol. 1999; 11:685–692.
Article6. Gille U, Salomon FV. Growth of the cloacal bursa (bursa of Fabricius) and spleen in ducks. Anat Histol Embryol. 1999; 28:229–233.7. Glick B. Embryogenesis of the bursa of Fabricius: Stem cell, microenvironment, and receptor-paracrine pathway. Poultry Sci. 1995; 74:419–426.
Article8. Glick B. Historical perspective: the bursa of Fabricius and its influence on B-cell development, past and present. Vet Immunol Immunopathol. 1991; 30:3–12.
Article9. Glick B, LaVia MF, Koger B. Flow cytometric analysis of bursal, thymic, and splenic cells from normal and cyclophosphamide-treated embryos. Poult Sci. 1985; 64:723–731.
Article10. Harrison L, Brown C, Afonso C, Zhang J, Susta L. Early occurrence of apoptosis in lymphoid tissues from chickens infected with strains of Newcastle disease virus of varying virulence. J Comp Pathol. 2011; 145:327–335.
Article11. Higgins SE, Berghman LR, Moore RW, Caldwell DJ, Caldwell DY, Tizard I, Hargis BM. In situ detection and quantification of bursa of Fabricius cellular proliferation or apoptosis in normal or steroid-treated neonatal chicks. Poult Sci. 2002; 81:1136–1141.
Article12. Hodges RD. The histology of the fowl. London: Academic Press;1974. p. 206–212.13. Kang Z, Bédécarrats GY, Zadworny D. Expression patterns of the prolactin receptor gene in chicken lymphoid tissues during embryogenesis and posthatch period. Poult Sci. 2007; 86:2404–2412.
Article14. Lampisuo M, Arstila TP, Liippo J, Lassila O. Expression of chL12 surface antigen is assoiciated with cell survival in the avian bursa of Fabricius. Scand J Immunol. 1998; 47:223–228.
Article15. Lassila O. Emigration of B cells from the chicken bursa of Fabricius. Eur J Immunol. 1989; 19:955–958.16. Lee RM, Gillet G, Neiman P. Molecular events in avian neoplasia: regulators of cell death in development of B-cell lymphomas in the chicken bursa of Fabricius. Avian Pathol. 1998; 27:S16–S20.
Article17. Li K, Kang XT, LiuY , Li M, Song GR. Study on ultrastructure of immune cells and natural apoptotic cells in central immune organs of Gushi chickens. Xu Mu Shou Yi Xue Bao. 2007; 38:89–95.18. Li K, Sun GR, Kang XT, Li CL, Liu Y, Liu ZH, Li M, Wang YC. Dynamic changes for cell proliferation in immune organs of Gushi chickens. Chin J Vet Sci. 2008; 8:953–956.19. Lydyard PM, Grossi CE, Cooper MD. Ontogeny of B cells in the chicken. I. Sequential development of clonal diversity in the bursa. J Exp Med. 1976; 144:79–97.
Article20. Mel'nikova VI, Afanas'eva MA, Sapozhnikov AM, Zakharova LA. Dynamics of apoptosis and proliferation in the rat thymus and spleen during perinatal development. Ontogenez. 2006; 37:286–291.21. Motyka B, Reynolds JD. Apoptosis is associated with the extensive B cell death in the sheep ileal Peyer's patch and the chicken bursa of Fabricius: a possible role in B cell selection. Eur J Immunol. 1991; 21:1951–1958.
Article22. Neiman PE, Thomas SJ, Loring G. Induction of apoptosis during normal and neoplastic B cell development in the bursa of Fabricius. Proc Natl Acad Sci U S A. 1991; 88:5857–5861.
Article23. Ojeda F, Skardova I, Guarda MI, Ulloa J, Folch H. Proliferation and apoptosis in infection with infectious bursal disease virus: a flow cytometric study. Avian Dis. 1997; 41:312–316.
Article24. Olah I, Glick B. Follicle-associated epithelium and medullary epithelial tissue of the bursa of Fabricius are two different compartments. Anat Rec. 1992; 233:577–587.
Article25. Paramithiotis E, Ratcliffe MJH. Bursa-dependent subpopulations of peripheral B lymphocytes in chicken blood. Eur J Immunol. 1993; 23:96–102.
Article26. Paramithiotis E, Ratcliffe MJH. B cell emigration directly from the cortex of lymphoid follicles in the bursa of Fabricius. Eur J Immunol. 1994; 24:458–463.
Article27. Peng X, Cui Y, Cui W, Deng J, Cui H, Yang F. The cell cycle arrest and apoptosis of bursa of Fabricius induced by low selenium in chickens. Biol Trace Elem Res. 2011; 139:32–40.
Article28. Resendes AR, Majó N, Segalés J, Espadamala J, Mateu E, Chianini F, Nofrarías M, Domingo M. Apoptosis in normal lymphoid organs from healthy normal, conventional pigs at different ages detected by TUNEL and cleaved caspase-3 immunohistochemistry in paraffin-embedded tissues. Vet Immunol Immunopathol. 2004; 99:203–213.
Article29. Rodríguez-Méndez AJ, Luna-Acosta JL, Carranza M, Harvey S, Arámburo C, Luna M. Growth hormone expression in stromal and non-stromal cells in the bursa of Fabricius during bursal development and involution: causal relationships? Gen Comp Endocrinol. 2010; 167:297–307.
Article30. Saifuddin M, Manktelow BW, Moriarty KM, Christensen NH, Birtles MJ. Age-related functional changes in the follicle-associated epithelium of the bursa of Fabricius in Shaver cockerels. N Z Vet J. 1988; 36:108–111.
Article31. Sayegh CE, Ratcliffe MJH. Perinatal deletion of B cells expressing surface Ig molecules that lack V(D) J-encoded determinants in the bursa of Fabricius is not due to intrafollicular competition. J Immunol. 2000; 164:5041–5048.
Article32. Weill JC, Reynaud CA. The chicken B cells compartment. Science. 1987; 238:1094–1098.33. Yuan G, Cheng A, Wang M, Han X, Zhou Y, Liu F. Preliminary study on duck enteritis virus-induced lymphocyte apoptosis in vivo. Avian Dis. 2007; 51:546–549.
Article34. Zhang S, Chen W, Yu Y, Bao E. Relationship between bcl-2 expression and apoptosis in chicken immune organs including adult and embryo. Nanjing Nong Ye Da Xue Xue Bao. 1999; 22:65–68.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of Cryptosporidium baileyi infection on the bursa of Fabricius in chickens
- Isolation and immunomodulatory activity of bursal peptide, a novel bursal peptide from the chicken bursa of Fabricius
- Effect of Cryptosporidium baileyi infection on antibody response to sRBC in chickens
- Effect of Diclazuril on the Bursa of Fabricius Morphology and SIgA Expression in Chickens Infected with Eimeria tenella
- Dexamethasone reduces infectious bursal disease mortality in chickens