Yonsei Med J.
2015 Mar;56(2):426-432. 10.3349/ymj.2015.56.2.426.
Effects of Interleukin-13 and Montelukast on the Expression of Zonula Occludens-1 in Human Podocytes
- Affiliations
-
- 1Department of Pediatrics, Ajou University School of Medicine, Suwon, Korea.
- 2Department of Pediatrics, Severance Children's Hospital, Yonsei University College of Medicine, Seoul, Korea. shinji@yuhs.ac
- 3Children's and Academic Renal Unit, Southmead Hospital, University of Bristol, Bristol, United Kingdom.
- 4Department of Pediatrics, Chungbuk National University College of Medicine, Cheongju, Korea. tsha@chungbuk.ac.kr
- KMID: 2070020
- DOI: http://doi.org/10.3349/ymj.2015.56.2.426
Abstract
- PURPOSE
The aim of this study was to investigate whether pathologic changes in zonula occludens-1 (ZO-1) are induced by interleukin-13 (IL-13) in the experimental minimal-change nephrotic syndrome (MCNS) model and to determine whether montelukast, a leukotriene receptor antagonist, has an effect on ZO-1 restoration in cultured human podocytes.
MATERIALS AND METHODS
Human podocytes cultured on bovine serum albumin-coated plates were treated with different doses of IL-13 and montelukast and then examined for distribution using confocal microscopy and for ZO-1 protein levels using Western blotting.
RESULTS
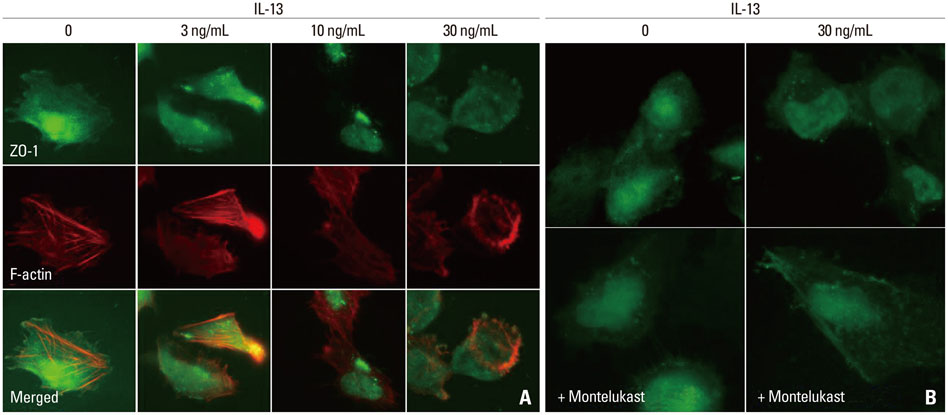
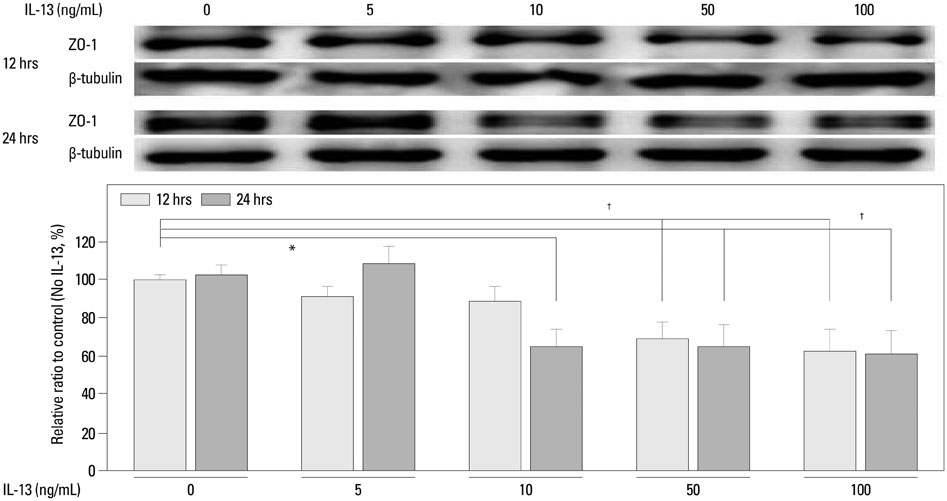
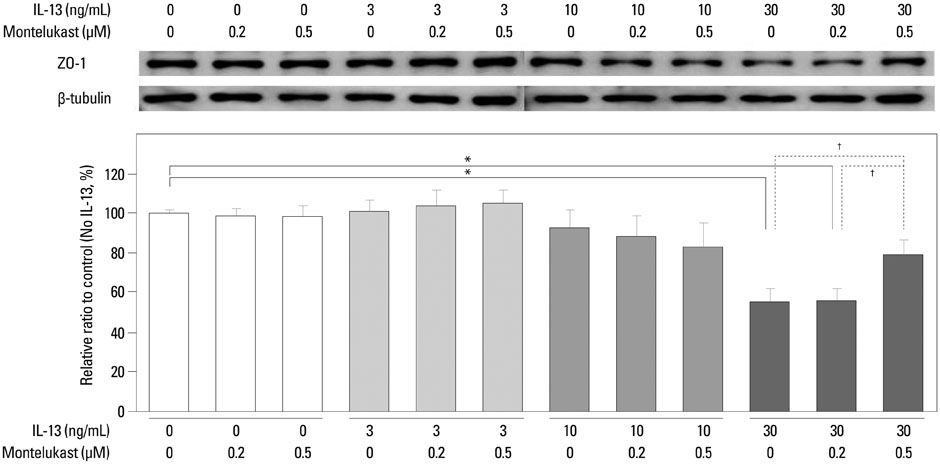
ZO-1 was internalized and shown to accumulate in the cytoplasm of human podocytes in an IL-13 dose-dependent manner. High doses (50 and 100 ng/mL) of IL-13 decreased the levels of ZO-1 protein at 12 and 24 h (both p<0.01; n=3), which were significantly reversed by a high dose (0.5 microM) montelukast treatment (p<0.01; n=3).
CONCLUSION
Our results suggest that IL-13 alters the expression of ZO-1, and such alterations in the content and distribution of ZO-1 may be relevant in the pathogenesis of proteinuria in the MCNS model.
MeSH Terms
-
Acetates/*pharmacology
Blotting, Western
Dose-Response Relationship, Drug
Humans
Interleukin-13/*pharmacology
Leukotriene Antagonists/*pharmacology
Microscopy, Confocal
Podocytes/*drug effects/metabolism
Proteinuria/pathology
Quinolines/*pharmacology
Tight Junctions
Zonula Occludens-1 Protein/*metabolism
Acetates
Interleukin-13
Leukotriene Antagonists
Quinolines
Zonula Occludens-1 Protein
Figure
Reference
-
1. Eddy AA, Symons JM. Nephrotic syndrome in childhood. Lancet. 2003; 362:629–639.
Article2. Kurihara H. [Molecular dynamics of proteins found exclusively in glomerular epithelial cells]. Rinsho Byori. 2000; 48:491–497.3. Asanuma K, Mundel P. The role of podocytes in glomerular pathobiology. Clin Exp Nephrol. 2003; 7:255–259.
Article4. Yap HK, Cheung W, Murugasu B, Sim SK, Seah CC, Jordan SC. Th1 and Th2 cytokine mRNA profiles in childhood nephrotic syndrome: evidence for increased IL-13 mRNA expression in relapse. J Am Soc Nephrol. 1999; 10:529–537.5. Cheung W, Wei CL, Seah CC, Jordan SC, Yap HK. Atopy, serum IgE, and interleukin-13 in steroid-responsive nephrotic syndrome. Pediatr Nephrol. 2004; 19:627–632.
Article6. Wei CL, Cheung W, Heng CK, Arty N, Chong SS, Lee BW, et al. Interleukin-13 genetic polymorphisms in Singapore Chinese children correlate with long-term outcome of minimal-change disease. Nephrol Dial Transplant. 2005; 20:728–734.
Article7. Lai KW, Wei CL, Tan LK, Tan PH, Chiang GS, Lee CG, et al. Overexpression of interleukin-13 induces minimal-change-like nephropathy in rats. J Am Soc Nephrol. 2007; 18:1476–1485.
Article8. Van Den Berg JG, Aten J, Chand MA, Claessen N, Dijkink L, Wijdenes J, et al. Interleukin-4 and interleukin-13 act on glomerular visceral epithelial cells. J Am Soc Nephrol. 2000; 11:413–422.
Article9. Nagafuchi A. Molecular architecture of adherens junctions. Curr Opin Cell Biol. 2001; 13:600–603.
Article10. Fanning AS, Anderson JM. Zonula occludens-1 and -2 are cytosolic scaffolds that regulate the assembly of cellular junctions. Ann N Y Acad Sci. 2009; 1165:113–120.
Article11. Fanning AS, Anderson JM. PDZ domains: fundamental building blocks in the organization of protein complexes at the plasma membrane. J Clin Invest. 1999; 103:767–772.
Article12. Van Itallie CM, Fanning AS, Bridges A, Anderson JM. ZO-1 stabilizes the tight junction solute barrier through coupling to the perijunctional cytoskeleton. Mol Biol Cell. 2009; 20:3930–3940.
Article13. Drazen JM, Israel E, O'Byrne PM. Treatment of asthma with drugs modifying the leukotriene pathway. N Engl J Med. 1999; 340:197–206.
Article14. Niimi A. Cough, asthma, and cysteinyl-leukotrienes. Pulm Pharmacol Ther. 2013; 26:514–519.
Article15. Bisgaard H. Pathophysiology of the cysteinyl leukotrienes and effects of leukotriene receptor antagonists in asthma. Allergy. 2001; 56 Suppl 66:7–11.
Article16. Forbes TA, Lunn AJ. Montelukast: a novel therapeutic option in eosinophilic peritonitis. Pediatr Nephrol. 2014; 29:1279–1282.
Article17. Kang MJ, Lee SY, Kim HB, Yu J, Kim BJ, Choi WA, et al. Association of IL-13 polymorphisms with leukotriene receptor antagonist drug responsiveness in Korean children with exercise-induced bronchoconstriction. Pharmacogenet Genomics. 2008; 18:551–558.
Article18. Saleem MA, O'Hare MJ, Reiser J, Coward RJ, Inward CD, Farren T, et al. A conditionally immortalized human podocyte cell line demonstrating nephrin and podocin expression. J Am Soc Nephrol. 2002; 13:630–638.
Article19. Ha TS, Song CJ, Lee JH. Effects of advanced glycosylation endproducts on perlecan core protein of glomerular epithelium. Pediatr Nephrol. 2004; 19:1219–1224.
Article20. Ha TS. High-glucose and advanced glycosylation end products increased podocyte permeability via PI3-K/Akt signaling. J Mol Med (Berl). 2010; 88:391–400.
Article21. Ha TS, Choi JY, Park HY, Lee JS. Ginseng total saponin improves podocyte hyperpermeability induced by high glucose and advanced glycosylation endproducts. J Korean Med Sci. 2011; 26:1316–1321.
Article22. Greka A, Mundel P. Cell biology and pathology of podocytes. Annu Rev Physiol. 2012; 74:299–323.
Article23. Mundel P, Shankland SJ. Podocyte biology and response to injury. J Am Soc Nephrol. 2002; 13:3005–3015.
Article24. Reiser J, Kriz W, Kretzler M, Mundel P. The glomerular slit diaphragm is a modified adherens junction. J Am Soc Nephrol. 2000; 11:1–8.
Article25. Comper WD, Glasgow EF. Charge selectivity in kidney ultrafiltration. Kidney Int. 1995; 47:1242–1251.
Article26. Goode NP, Shires M, Davison AM. The glomerular basement membrane charge-selectivity barrier: an oversimplified concept? Nephrol Dial Transplant. 1996; 11:1714–1716.
Article27. Hartsock A, Nelson WJ. Adherens and tight junctions: structure, function and connections to the actin cytoskeleton. Biochim Biophys Acta. 2008; 1778:660–669.
Article28. Schnabel E, Anderson JM, Farquhar MG. The tight junction protein ZO-1 is concentrated along slit diaphragms of the glomerular epithelium. J Cell Biol. 1990; 111:1255–1263.
Article29. Kurihara H, Anderson JM, Farquhar MG. Increased Tyr phosphorylation of ZO-1 during modification of tight junctions between glomerular foot processes. Am J Physiol. 1995; 268:F514–F524.
Article30. Itoh M, Furuse M, Morita K, Kubota K, Saitou M, Tsukita S. Direct binding of three tight junction-associated MAGUKs, ZO-1, ZO-2, and ZO-3, with the COOH termini of claudins. J Cell Biol. 1999; 147:1351–1363.
Article31. Luimula P, Ahola H, Wang SX, Solin ML, Aaltonen P, Tikkanen I, et al. Nephrin in experimental glomerular disease. Kidney Int. 2000; 58:1461–1468.
Article32. Luimula P, Sandström N, Novikov D, Holthöfer H. Podocyte-associated molecules in puromycin aminonucleoside nephrosis of the rat. Lab Invest. 2002; 82:713–718.
Article33. Raats CJ, van den Born J, Bakker MA, Oppers-Walgreen B, Pisa BJ, Dijkman HB, et al. Expression of agrin, dystroglycan, and utrophin in normal renal tissue and in experimental glomerulopathies. Am J Pathol. 2000; 156:1749–1765.
Article34. Kawachi H, Kurihara H, Topham PS, Brown D, Shia MA, Orikasa M, et al. Slit diaphragm-reactive nephritogenic MAb 5-1-6 alters expression of ZO-1 in rat podocytes. Am J Physiol. 1997; 273:F984–F993.35. Kurihara H, Anderson JM, Kerjaschki D, Farquhar MG. The altered glomerular filtration slits seen in puromycin aminonucleoside nephrosis and protamine sulfate-treated rats contain the tight junction protein ZO-1. Am J Pathol. 1992; 141:805–816.36. Matter K, Balda MS. Signalling to and from tight junctions. Nat Rev Mol Cell Biol. 2003; 4:225–236.
Article37. Kim BS, Park HC, Kang SW, Choi KH, Ha SK, Han DS, et al. Impact of cyclosporin on podocyte ZO-1 expression in puromycin aminonucleoside nephrosis rats. Yonsei Med J. 2005; 46:141–148.
Article38. Macconi D, Ghilardi M, Bonassi ME, Mohamed EI, Abbate M, Colombi F, et al. Effect of angiotensin-converting enzyme inhibition on glomerular basement membrane permeability and distribution of zonula occludens-1 in MWF rats. J Am Soc Nephrol. 2000; 11:477–489.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Expression of Osteopontin, ZO-1 and E-cadherin in Adenoma and Adenocarcinoma of the Colon
- Interleukin-13 Increases Podocyte Apoptosis in Cultured Human Podocytes
- Effects of Angiotensin II on ZO-1 in Glomerular Epithelial Cells
- Hypoxia Increases Epithelial Permeability in Human Nasal Epithelia
- Chlorogenic Acid Decreases Retinal Vascular Hyperpermeability in Diabetic Rat Model