Korean J Radiol.
2015 Feb;16(1):146-153. 10.3348/kjr.2015.16.1.146.
Pelvic Solitary Plasmacytoma: Computed Tomography and Magnetic Resonance Imaging Findings with Histopathologic Correlation
- Affiliations
-
- 1Department of Radiology, Second Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou 310009, China. cjr.yurisheng@vip.163.com
- 2Zhejiang University School of Medicine, Hangzhou 310012, China.
- 3Department of Pathology, Second Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou 310009, China.
- KMID: 2069993
- DOI: http://doi.org/10.3348/kjr.2015.16.1.146
Abstract
OBJECTIVE
To describe the imaging features of pelvic solitary plasmacytoma and to correlate them with the pathologic grade.
MATERIALS AND METHODS
A retrospective study was performed on the imaging features of 10 patients with a histological diagnosis of pelvic solitary plasmacytoma. The imaging studies were assessed for bone expansion, cortical destruction, signal intensity/density of soft tissue mass and enhancement manifestations, which were then correlated to the pathologic grade.
RESULTS
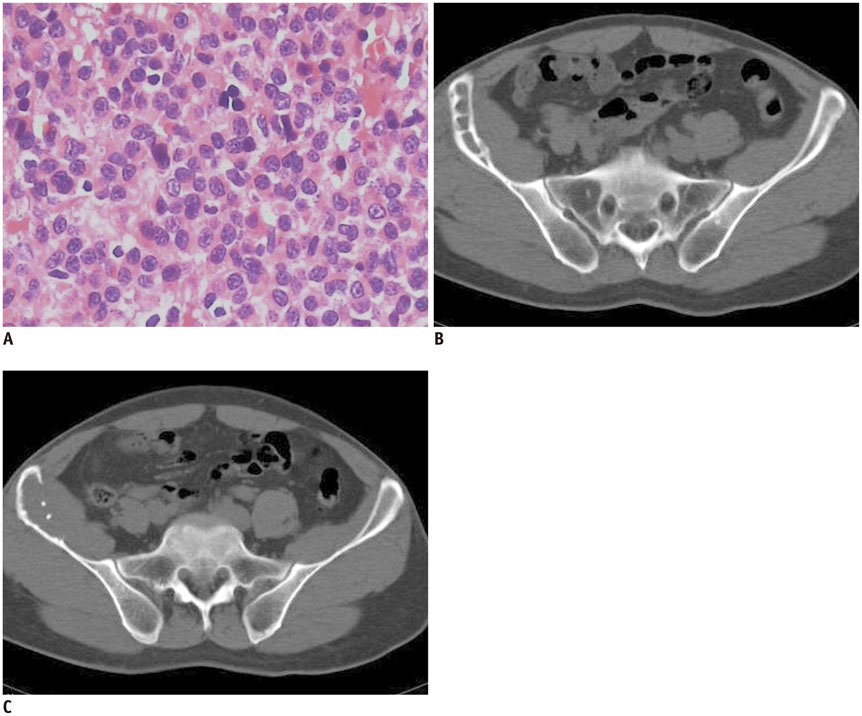
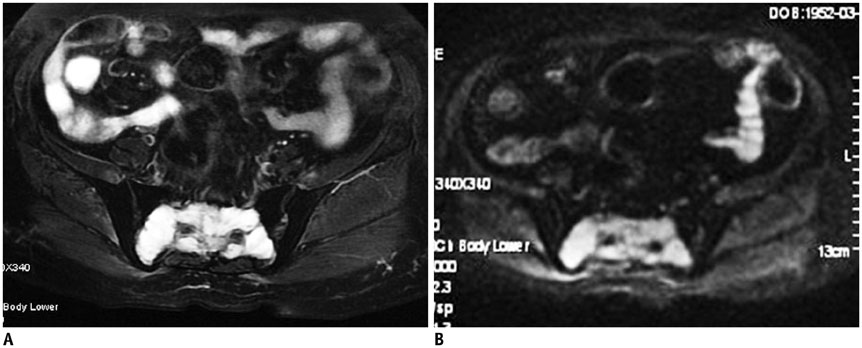
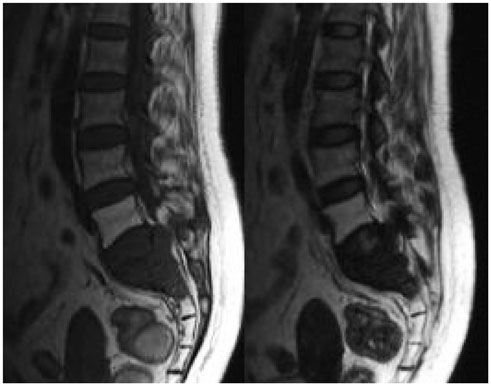
The imaging features of pelvic solitary plasmacytoma revealed 3 different types: multilocular type (n = 5), unilocular type (n = 2) and complete osteolytic destruction type (n = 3) on computed tomography and MRI. Pathologically, the tumors were classified into low, intermediate and high grades. Features such as multilocular change, perilesional osteosclerosis, slight expansion, local bone cortex disruptions and masses inside bone destruction, often suggest a low-grade solitary plasmacytoma; complete osteolytic destruction, huge soft tissue mass, and osseous defects imply a higher pathologic grade.
CONCLUSION
Pelvic solitary plasmacytoma has various imaging manifestations, while a slight expansile osteolytic feature with multilocular change or homogeneous enhancement highly suggests its diagnosis. The distinctive imaging features of pelvic solitary plasmacytoma are well correlated to the pathologic grade.
Keyword
MeSH Terms
Figure
Reference
-
1. Ellis PA, Colls BM. Solitary plasmacytoma of bone: clinical features, treatment and survival. Hematol Oncol. 1992; 10:207–211.2. Kilciksiz S, Karakoyun-Celik O, Agaoglu FY, Haydaroglu A. A review for solitary plasmacytoma of bone and extramedullary plasmacytoma. ScientificWorldJournal. 2012; 2012:895765.3. Soutar R, Lucraft H, Jackson G, Reece A, Bird J, Low E, et al. Guidelines on the diagnosis and management of solitary plasmacytoma of bone and solitary extramedullary plasmacytoma. Clin Oncol (R Coll Radiol). 2004; 16:405–413.4. Dimopoulos MA, Moulopoulos LA, Maniatis A, Alexanian R. Solitary plasmacytoma of bone and asymptomatic multiple myeloma. Blood. 2000; 96:2037–2044.5. Pasch W, Zhao X, Rezk SA. Solitary plasmacytoma of the bone involving young individuals, is there a role for preceding trauma. Int J Clin Exp Pathol. 2012; 5:463–467.6. Holland J, Trenkner DA, Wasserman TH, Fineberg B. Plasmacytoma. Treatment results and conversion to myeloma. Cancer. 1992; 69:1513–1517.7. Susnerwala SS, Shanks JH, Banerjee SS, Scarffe JH, Farrington WT, Slevin NJ. Extramedullary plasmacytoma of the head and neck region: clinicopathological correlation in 25 cases. Br J Cancer. 1997; 75:921–927.8. Suh YG, Suh CO, Kim JS, Kim SJ, Pyun HO, Cho J. Radiotherapy for solitary plasmacytoma of bone and soft tissue: outcomes and prognostic factors. Ann Hematol. 2012; 91:1785–1793.9. Kosaka N, Maeda M, Uematsu H, Matsumine A, Koshimoto Y, Itoh H. Solitary plasmacytoma of the sacrum. Radiologic findings of three cases. Clin Imaging. 2005; 29:426–429.10. Wong CL, Mansberg R. Solitary plasmacytoma of bone: an unusual cause of severe sacral pain in a young man. Clin Nucl Med. 2005; 30:612–614.11. Llauger J, Palmer J, Amores S, Bagué S, Camins A. Primary tumors of the sacrum: diagnostic imaging. AJR Am J Roentgenol. 2000; 174:417–424.12. Diel J, Ortiz O, Losada RA, Price DB, Hayt MW, Katz DS. The sacrum: pathologic spectrum, multimodality imaging, and subspecialty approach. Radiographics. 2001; 21:83–104.13. Sabattini E, Bacci F, Sagramoso C, Pileri SA. WHO classification of tumours of haematopoietic and lymphoid tissues in 2008: an overview. Pathologica. 2010; 102:83–87.14. Hughes M, Soutar R, Lucraft H, Owen R, Bird J. Guidelines on the diagnosis and management of solitary plasmacytoma of bone, extramedullary plasmacytoma and multiple solitary plasmacytomas: 2009 update. London: British Committee for Standards in Haematology;Accessed October 14, 2013. Web site. http://www.guideline.gov/content.aspx?id=15514.15. Bartl R, Frisch B, Fateh-Moghadam A, Kettner G, Jaeger K, Sommerfeld W. Histologic classification and staging of multiple myeloma. A retrospective and prospective study of 674 cases. Am J Clin Pathol. 1987; 87:342–355.16. Major NM, Helms CA, Richardson WJ. The "mini brain": plasmacytoma in a vertebral body on MR imaging. AJR Am J Roentgenol. 2000; 175:261–263.17. Ooi GC, Chim JC, Au WY, Khong PL. Radiologic manifestations of primary solitary extramedullary and multiple solitary plasmacytomas. AJR Am J Roentgenol. 2006; 186:821–827.18. Glazer HS, Niemeyer JH, Balfe DM, Hayden RE, Emami B, Devineni VR, et al. Neck neoplasms: MR imaging. Part II. Posttreatment evaluation. Radiology. 1986; 160:349–354.19. Kirsch J, Ilaslan H, Bauer TW, Sundaram M. The incidence of imaging findings, and the distribution of skeletal lymphoma in a consecutive patient population seen over 5 years. Skeletal Radiol. 2006; 35:590–594.20. Taki S, Kakuda K, Kakuma K, Yamashita R, Kosugi M, Annen Y. Posterior mediastinal chordoma: MR imaging findings. AJR Am J Roentgenol. 1996; 166:26–27.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A study on the comparision of various imaging methods for the staging of renal cell carcinoma
- Solitary Plasmacytoma of the Skull Base: Case Report
- Solitary Dural Plasmacytoma: Case Report
- Pelvic Hydatid Disease: CT and MRI Findings Causing Sciatica
- Solitary Extramedullary Plasmacytoma of the Liver without Systemic Monoclonal Gammopathy