J Korean Med Sci.
2014 Sep;29(Suppl 2):S139-S145. 10.3346/jkms.2014.29.S2.S139.
Cobalt Chloride Attenuates Oxidative Stress and Inflammation through NF-kappaB Inhibition in Human Renal Proximal Tubular Epithelial Cells
- Affiliations
-
- 1Department of Internal Medicine, Inje University College of Medicine, Ilsan Paik Hospital, Seoul, Korea.
- 2Department of Internal Medicine, Seoul National University Bundang Hospital, Seongnam, Korea. kyna@snubh.org
- 3Department of Internal Medicine, Seoul National University College of Medicine, Seoul, Korea.
- KMID: 2069805
- DOI: http://doi.org/10.3346/jkms.2014.29.S2.S139
Abstract
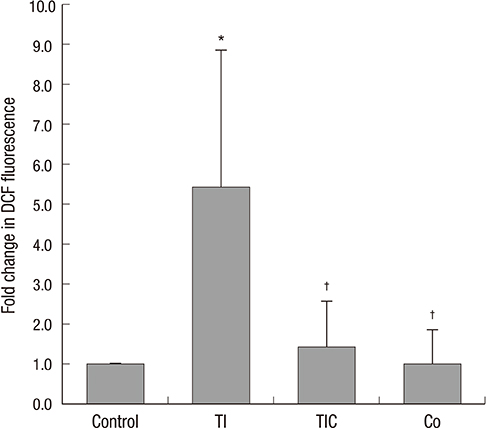
- We evaluated the effect of cobalt chloride (CoCl2) on TNF-alpha and IFN-gamma-induced-inflammation and reactive oxygen species (ROS) in renal tubular epithelial cells (HK-2 cells). We treated HK-2 cells with CoCl2 before the administration of TNF-alpha/IFN-gamma. To regulate hemeoxygenase-1 (HO-1) expression, the cells were treated CoCl2 or HO-1 siRNA. CoCl2 reduced the generation of ROS induced by TNF-alpha/IFN-gamma. TNF-alpha/IFN-gamma-treated-cells showed an increase in the nuclear translocation of phosphorylated NF-kappaBp65 protein, the DNA-binding activity of NF-kappaBp50 and NF-kappaB transcriptional activity and a decrease in IkappaBalpha protein expression. These changes were restored by CoCl2. We noted an intense increase in monocyte chemoattractant protein-1 (MCP-1) and regulated on activation normal T cell expressed and secreted (RANTES) production in TNF-alpha/IFN-gamma-treated cells. We demonstrated that this effect was mediated through NF-kappaB signaling because an NF-kappaB inhibitor significantly reduced MCP-1 and RANTES production. CoCl2 effectively reduced MCP-1 and RANTES production. The expression of HO-1 was increased by CoCl2 and decreased by HO-1 siRNA. However, knockdown of HO-1 by RNA interference did not affect MCP-1 or RANTES production. We suggest that CoCl2 has a protective effect on TNF-alpha/IFN-gamma-induced inflammation through the inhibition of NF-kappaB and ROS in HK-2 cells. However, CoCl2 appears to act in an HO-1-independent manner.
Keyword
MeSH Terms
-
Cell Line
Chemokine CCL2/metabolism
Chemokine CCL5/metabolism
Cobalt/*pharmacology
Epithelial Cells/cytology/metabolism
Heme Oxygenase-1/antagonists & inhibitors/genetics/metabolism
Humans
*Inflammation
Interferon-gamma/pharmacology
Kidney Tubules, Proximal/cytology
NF-kappa B/antagonists & inhibitors/genetics/*metabolism
NF-kappa B p50 Subunit/genetics/metabolism
Oxidative Stress/*drug effects
Phosphorylation
Protein Binding
RNA Interference
RNA, Small Interfering/metabolism
Transcription Factor RelA/metabolism
Tumor Necrosis Factor-alpha/pharmacology
Chemokine CCL2
Chemokine CCL5
Cobalt
Heme Oxygenase-1
Interferon-gamma
NF-kappa B
NF-kappa B p50 Subunit
RNA, Small Interfering
Transcription Factor RelA
Tumor Necrosis Factor-alpha
Figure
Reference
-
1. Deckers JG, Van Der Woude FJ, Van Der Kooij SW, Daha MR. Synergistic effect of IL-1alpha, IFN-gamma, and TNF-alpha on RANTES production by human renal tubular epithelial cells in vitro. J Am Soc Nephrol. 1998; 9:194–202.2. Ernandez T, Mayadas TN. Immunoregulatory role of TNF alpha in inflammatory kidney diseases. Kidney Int. 2009; 76:262–276.3. Pfeffer K. Biological functions of tumor necrosis factor cytokines and their receptors. Cytokine Growth Factor Rev. 2003; 14:185–191.4. Lee BH, Lee TJ, Jung JW, Oh DJ, Choi JC, Shin JW, Park IW, Choi BW, Kim JY. The role of keratinocyte-derived chemokine in hemorrhage-induced acute lung injury in mice. J Korean Med Sci. 2009; 24:775–781.5. Gibbs LS, Lai L, Malik AB. Tumor necrosis factor enhances the neutrophil-dependent increase in endothelial permeability. J Cell Physiol. 1990; 145:496–500.6. Lee IT, Luo SF, Lee CW, Wang SW, Lin CC, Chang CC, Chen YL, Chau LY, Yang CM. Overexpression of HO-1 protects against TNF-alpha-mediated airway inflammation by down-regulation of TNFR1-dependent oxidative stress. Am J Pathol. 2009; 175:519–532.7. Woo CH, Eom YW, Yoo MH, You HJ, Han HJ, Song WK, Yoo YJ, Chun JS, Kim JH. Tumor necrosis factor-alpha generates reactive oxygen species via a cytosolic phospholipase A2-linked cascade. J Biol Chem. 2000; 275:32357–32362.8. Sanz AB, Sanchez-Niño MD, Ramos AM, Moreno JA, Santamaria B, Ruiz-Ortega M, Egido J, Ortiz A. NF-kappaB in renal inflammation. J Am Soc Nephrol. 2010; 21:1254–1262.9. Kim KS, Rajagopal V, Gonsalves C, Johnson C, Kalra VK. A novel role of hypoxia-inducible factor in cobalt chloride- and hypoxia-mediated expression of IL-8 chemokine in human endothelial cells. J Immunol. 2006; 177:7211–7224.10. Wan X, Yang J, Xing L, Fan L, Hu B, Chen X, Cao C. Inhibition of IκB Kinase β attenuates hypoxia-induced inflammatory mediators in rat renal tubular cells. Transplant Proc. 2011; 43:1503–1510.11. Maines MD, Kappas A. Studies on the mechanism of induction of haem oxygenase by cobalt and other metal ions. Biochem J. 1976; 154:125–131.12. Nath KA. Heme oxygenase-1: a redoubtable response that limits reperfusion injury in the transplanted adipose liver. J Clin Invest. 1999; 104:1485–1486.13. Choi AM, Alam J. Heme oxygenase-1: function, regulation, and implication of a novel stress-inducible protein in oxidant-induced lung injury. Am J Respir Cell Mol Biol. 1996; 15:9–19.14. Murali NS, Ackerman AW, Croatt AJ, Cheng J, Grande JP, Sutor SL, Bram RJ, Bren GD, Badley AD, Alam J, et al. Renal upregulation of HO-1 reduces albumin-driven MCP-1 production: implications for chronic kidney disease. Am J Physiol Renal Physiol. 2007; 292:F837–F844.15. Ahn JM, You SJ, Lee YM, Oh SW, Ahn SY, Kim S, Chin HJ, Chae DW, Na KY. Hypoxia-inducible factor activation protects the kidney from gentamicin-induced acute injury. PLoS One. 2012; 7:e48952.16. Basak S, Hoffmann A. Crosstalk via the NF kappaB signaling system. Cytokine Growth Factor Rev. 2008; 19:187–197.17. Hayden MS, Ghosh S. Shared principles in NF-kappaB signaling. Cell. 2008; 132:344–362.18. Perkins ND. Integrating cell-signalling pathways with NF-kappaB and IKK function. Nat Rev Mol Cell Biol. 2007; 8:49–62.19. Wan F, Lenardo MJ. Specification of DNA binding activity of NF-kappaB proteins. Cold Spring Harb Perspect Biol. 2009; 1:a000067.20. Haas AL. Linear polyubiquitylation: the missing link in NF-kappaB signalling. Nat Cell Biol. 2009; 11:116–118.21. Häussler U, von Wichert G, Schmid RM, Keller F, Schneider G. Epidermal growth factor activates nuclear factor-kappaB in human proximal tubule cells. Am J Physiol Renal Physiol. 2005; 289:F808–F815.22. Zhang Z, Yuan W, Sun L, Szeto FL, Wong KE, Li X, Kong J, Li YC. 1,25-Dihydroxyvitamin D3 targeting of NF-kappaB suppresses high glucose-induced MCP-1 expression in mesangial cells. Kidney Int. 2007; 72:193–201.23. Mezzano SA, Barría M, Droguett MA, Burgos ME, Ardiles LG, Flores C, Egido J. Tubular NF-kappaB and AP-1 activation in human proteinuric renal disease. Kidney Int. 2001; 60:1366–1377.24. Sakai N, Wada T, Furuichi K, Iwata Y, Yoshimoto K, Kitagawa K, Kokubo S, Kobayashi M, Takeda S, Kida H, et al. p38 MAPK phosphorylation and NF-kappa B activation in human crescentic glomerulonephritis. Nephrol Dial Transplant. 2002; 17:998–1004.25. Takase O, Hirahashi J, Takayanagi A, Chikaraishi A, Marumo T, Ozawa Y, Hayashi M, Shimizu N, Saruta T. Gene transfer of truncated IkappaB alpha prevents tubulointerstitial injury. Kidney Int. 2003; 63:501–513.26. Höcherl K, Schmidt C, Kurt B, Bucher M. Inhibition of NF-kappaB ameliorates sepsis-induced downregulation of aquaporin-2/V2 receptor expression and acute renal failure in vivo. Am J Physiol Renal Physiol. 2010; 298:F196–F204.27. da Silva JL, Zand BA, Yang LM, Sabaawy HE, Lianos E, Abraham NG. Heme oxygenase isoform-specific expression and distribution in the rat kidney. Kidney Int. 2001; 59:1448–1457.28. Morimoto K, Ohta K, Yachie A, Yang Y, Shimizu M, Goto C, Toma T, Kasahara Y, Yokoyama H, Miyata T, et al. Cytoprotective role of heme oxygenase(HO)-1 in human kidney with various renal diseases. Kidney Int. 2001; 60:1858–1866.29. Nath KA. Renal response to repeated exposure to endotoxin: implications for acute kidney injury. Kidney Int. 2007; 71:477–479.30. Nath KA. Heme oxygenase-1: a provenance for cytoprotective pathways in the kidney and other tissues. Kidney Int. 2006; 70:432–443.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Dual effect of oxidative stress on NF-kappaB activation in HeLa cells
- Carbon monoxide releasing molecule-2 protects mice against acute kidney injury through inhibition of ER stress
- Hyperglycemia-induced Activation of Nuclear Transcription Factor kappaB in Cultured Fibroblasts and Endothelial Cells
- Differential Regulation of NF-kappaB Signaling during Human Cytomegalovirus Infection
- Effect of Endothelin-1 on the Expression of Monocyte Chemoattractant Protein-1 in Cultured Human Proximal Tubular Epithelial Cells