Yonsei Med J.
2014 May;55(3):725-731. 10.3349/ymj.2014.55.3.725.
Whole Blood Interferon-gamma Release Assay Is Insufficient for the Diagnosis of Sputum Smear Negative Pulmonary Tuberculosis
- Affiliations
-
- 1Department of Internal Medicine, Yonsei University College of Medicine, Seoul, Korea. yschang@yuhs.ac
- KMID: 2068683
- DOI: http://doi.org/10.3349/ymj.2014.55.3.725
Abstract
- PURPOSE
We investigated the value of an interferon-gamma release assay (IGRA) for the diagnosis of active pulmonary tuberculosis (PTB) among sputum smear negative PTB suspects in an environment with intermediate burden of PTB and high Bacillus Calmette-Guerin (BCG) vaccination rate.
MATERIALS AND METHODS
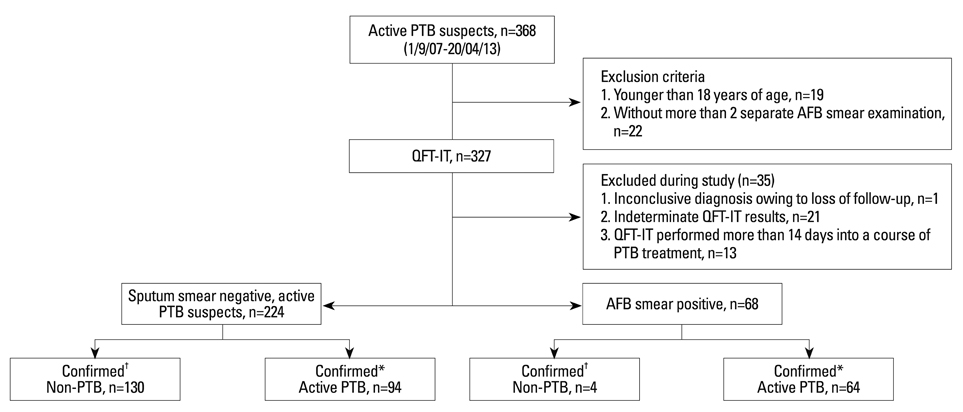
We retrospectively reviewed IGRA, medical records, chest PA and CT scan of PTB suspects seen at Gangnam Severance Hospital, Seoul, Korea from Oct. 2007 to Apr. 2013. "Active PTB" was diagnosed when 1) M. tuberculosis culture positive, 2) confirmation by pathologic examination; or 3) clinical findings compatible with TB.
RESULTS
Of 224 sputum smear negative PTB suspects, 94 were confirmed as having active PTB. There were no statistically significant differences in the diagnostic yield of IGRA between immunocompromised and immunocompetent sputum smear negative PTB suspects. IGRA did show superior sensitivity [81.9%, 95% confidence interval (CI); 74.13-89.70%] in the diagnosis of sputum smear negative PTB when compared with chest high-resolution computed tomography (HRCT), tuberculin skin test (TST), and chest X-ray (p<0.001). Also, IGRA showed highest negative predictive value (82.7%, 95% CI; 75.16-90.15%) when compared with HRCT, TST and chest X-ray (p=0.023). However, combining the results of IGRA with those of HRCT, TST, or both did not increase any diagnostic parameters.
CONCLUSION
Failure to increase diagnostic yields by combination with other diagnostic modalities suggests that additional enforcement with IGRA may be insufficient to exclude other diagnoses in sputum smear negative PTB suspects and to screen active PTB in an environment with intermediate TB prevalence and a high BCG vaccination rate.
Keyword
MeSH Terms
Figure
Reference
-
1. WHO. Global Tuberculosis Control: WHO Report 2010. Geneva: World Health Organizaion;2010.2. Nair N, Wares F, Sahu S. Tuberculosis in the WHO South-East Asia Region. Bull World Health Organ. 2010; 88:164.
Article3. KNTA. Association KNT. Trend of case notification rate per 100,000 by year, 2003-2009. 2010. Available at: https://www.knta.or.kr/inform/sub_03_10.asp.4. Foulds J, O'Brien R. New tools for the diagnosis of tuberculosis: the perspective of developing countries. Int J Tuberc Lung Dis. 1998; 2:778–783.5. Kanaya AM, Glidden DV, Chambers HF. Identifying pulmonary tuberculosis in patients with negative sputum smear results. Chest. 2001; 120:349–355.
Article6. Richeldi L. An update on the diagnosis of tuberculosis infection. Am J Respir Crit Care Med. 2006; 174:736–742.
Article7. Brock I, Weldingh K, Lillebaek T, Follmann F, Andersen P. Comparison of tuberculin skin test and new specific blood test in tuberculosis contacts. Am J Respir Crit Care Med. 2004; 170:65–69.
Article8. Samb B, Henzel D, Daley CL, Mugusi F, Niyongabo T, Mlika-Cabanne N, et al. Methods for diagnosing tuberculosis among in-patients in eastern Africa whose sputum smears are negative. Int J Tuberc Lung Dis. 1997; 1:25–30.9. Tozkoparan E, Deniz O, Ciftci F, Bozkanat E, Bicak M, Mutlu H, et al. The roles of HRCT and clinical parameters in assessing activity of suspected smear negative pulmonary tuberculosis. Arch Med Res. 2005; 36:166–170.
Article10. Leung EC, Leung CC, Leung WW, Kam KM, Yew WW, Lee SN, et al. Role of whole-blood interferon-gamma release assay in the diagnosis of smear-negative tuberculosis. Int J Tuberc Lung Dis. 2010; 14:1564–1570.11. Syed Ahamed Kabeer B, Raman B, Thomas A, Perumal V, Raja A. Role of QuantiFERON-TB gold, interferon gamma inducible protein-10 and tuberculin skin test in active tuberculosis diagnosis. PLoS One. 2010; 5:e9051.
Article12. Metcalfe JZ, Everett CK, Steingart KR, Cattamanchi A, Huang L, Hopewell PC, et al. Interferon-γ release assays for active pulmonary tuberculosis diagnosis in adults in low- and middle-income countries: systematic review and meta-analysis. J Infect Dis. 2011; 204:Suppl 4. S1120–S1129.
Article13. Sester M, Sotgiu G, Lange C, Giehl C, Girardi E, Migliori GB, et al. Interferon-γ release assays for the diagnosis of active tuberculosis: a systematic review and meta-analysis. Eur Respir J. 2011; 37:100–111.
Article14. Kim EY, Lim JE, Jung JY, Son JY, Lee KJ, Yoon YW, et al. Performance of the tuberculin skin test and interferon-gamma release assay for detection of tuberculosis infection in immunocompromised patients in a BCG-vaccinated population. BMC Infect Dis. 2009; 9:207.15. Tessema TA, Bjune G, Assefa G, Bjorvat B. An evaluation of the diagnostic value of clinical and radiological manifestations in patients attending the addis ababa tuberculosis centre. Scand J Infect Dis. 2001; 33:355–361.
Article16. Lee HM, Shin JW, Kim JY, Park IW, Choi BW, Choi JC, et al. HRCT and whole-blood interferon-gamma assay for the rapid diagnosis of smear-negative pulmonary tuberculosis. Respiration. 2010; 79:454–460.
Article17. Kang YA, Lee HW, Yoon HI, Cho B, Han SK, Shim YS, et al. Discrepancy between the tuberculin skin test and the whole-blood interferon gamma assay for the diagnosis of latent tuberculosis infection in an intermediate tuberculosis-burden country. JAMA. 2005; 293:2756–2761.
Article18. Aichelburg MC, Rieger A, Breitenecker F, Pfistershammer K, Tittes J, Eltz S, et al. Detection and prediction of active tuberculosis disease by a whole-blood interferon-gamma release assay in HIV-1-infected individuals. Clin Infect Dis. 2009; 48:954–962.
Article19. Zwerling A, Behr MA, Verma A, Brewer TF, Menzies D, Pai M. The BCG World Atlas: a database of global BCG vaccination policies and practices. PLoS Med. 2011; 8:e1001012.
Article20. Tsiouris SJ, Coetzee D, Toro PL, Austin J, Stein Z, El-Sadr W. Sensitivity analysis and potential uses of a novel gamma interferon release assay for diagnosis of tuberculosis. J Clin Microbiol. 2006; 44:2844–2850.
Article21. Dewan PK, Grinsdale J, Kawamura LM. Low sensitivity of a whole-blood interferon-gamma release assay for detection of active tuberculosis. Clin Infect Dis. 2007; 44:69–73.
Article22. Roh IS, Cho S, Eum SY, Cho SN. Kinetics of IFN-gamma and TNF-alpha gene expression and their relationship with disease progression after infection with Mycobacterium tuberculosis in guinea pigs. Yonsei Med J. 2013; 54:707–714.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Interferon-gamma Release Assay Using Pericardial Fluid and Peripheral Blood for the Diagnosis of Tuberculous Pericarditis: A Case Report
- The Usefulness of Whole-blood Interferon-gamma Release Assay for the Diagnosis of Extra-pulmonary Tuberculosis
- Diagnostic Utility of a Rapid ICT Tuberculosis Assay for the Diagnosis of Pulmonary Tuberculosis
- Comparison of Induced Sputum and Bronchoscopy in Diagnosis of Active Pulmonary Tuberculosis
- Diagnosis and treatment of latent tuberculosis infection