Yonsei Med J.
2014 May;55(3):700-708. 10.3349/ymj.2014.55.3.700.
The Role of Insulin Resistance in Diabetic Neuropathy in Koreans with Type 2 Diabetes Mellitus: A 6-Year Follow-Up Study
- Affiliations
-
- 1Department of Neurology, Yonsei University College of Medicine, Seoul, Korea. ycchoi@yuhs.ac
- 2Department of Neurology, Konyang University College of Medicine, Daejeon, Korea.
- 3Department of Neurology, Wonkwang University College of Medicine, Gunpo, Korea.
- 4Department of Neurology, Ewha Womans University College of Medicine, Seoul, Korea.
- 5Department of Neurology, Yonsei University College of Medicine, Yongin, Korea.
- 6Department of Internal Medicine, Yonsei University College of Medicine, Seoul, Korea.
- KMID: 2068680
- DOI: http://doi.org/10.3349/ymj.2014.55.3.700
Abstract
- PURPOSE
We previously reported that insulin resistance, low high-density lipoprotein (HDL) cholesterol, and glycaemic exposure Index are independently associated with peripheral neuropathy in Korean patients with type 2 diabetes mellitus. We followed the patients who participated in that study in 2006 for another 6 years to determine the relationship between insulin resistance and neuropathy.
MATERIALS AND METHODS
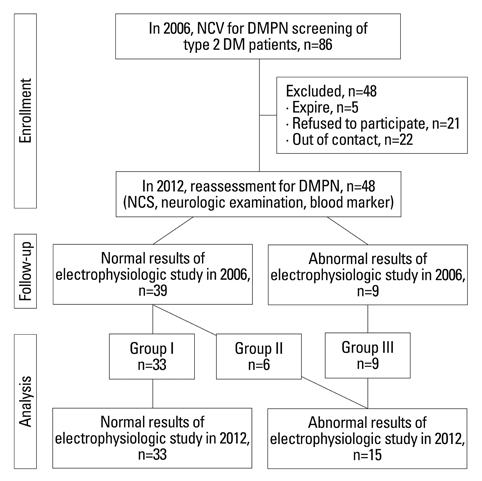
This study involved 48 of the original 86 Korean patients with type 2 diabetes mellitus who were referred to the Neurology clinic for the assessment of diabetic neuropathy from January 2006 to December 2006. These 48 patients received management for glycaemic control and prevention of diabetic complications in the outpatient clinic up to 2012. We reviewed blood test results and the nerve conduction study findings of these patients, taken over a 6-year period.
RESULTS
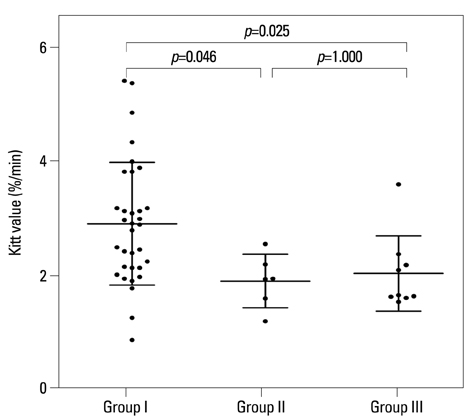
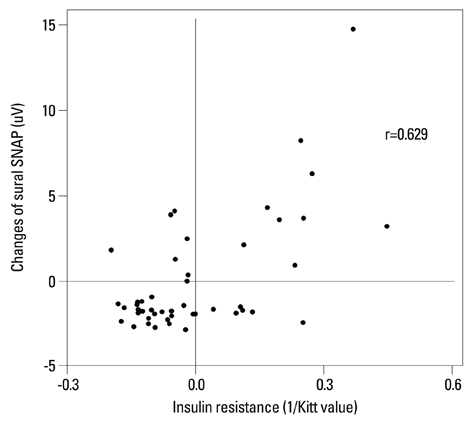
Low HDL cholesterol and high triglycerides significantly influenced the development of diabetic neuropathy. Kitt value (1/insulin resistance) in the previous study affected the occurrence of neuropathy, despite adequate glycaemic control with HbA1c <7%. Insulin resistance affected the development of diabetic neuropathy after 6 years: insulin resistance in 2006 showed a positive correlation with a change in sural sensory nerve action potential in 2012.
CONCLUSION
Diabetic neuropathy can be affected by previous insulin resistance despite regular glycaemic control. Dyslipidaemia should be controlled in patients who show high insulin resistance because HDL cholesterol and triglycerides are strongly correlated with later development of diabetic neuropathy.
MeSH Terms
Figure
Reference
-
1. Bruce KD, Hanson MA. The developmental origins, mechanisms, and implications of metabolic syndrome. J Nutr. 2010; 140:648–652.
Article2. Petersen KF, Shulman GI. Etiology of insulin resistance. Am J Med. 2006; 119:5 Suppl 1. S10–S16.
Article3. Kim DJ, Song KE, Park JW, Cho HK, Lee KW, Huh KB. Clinical characteristics of Korean type 2 diabetic patients in 2005. Diabetes Res Clin Pract. 2007; 77:Suppl 1. S252–S257.
Article4. Lee KO, Nam JS, Ahn CW, Hong JM, Kim SM, Sunwoo IN, et al. Insulin resistance is independently associated with peripheral and autonomic neuropathy in Korean type 2 diabetic patients. Acta Diabetol. 2012; 49:97–103.
Article5. Bril V. NIS-LL: the primary measurement scale for clinical trial endpoints in diabetic peripheral neuropathy. Eur Neurol. 1999; 41:Suppl 1. 8–13.
Article6. Dyck PJ, Lais A, Karnes JL, O'Brien P, Rizza R. Fiber loss is primary and multifocal in sural nerves in diabetic polyneuropathy. Ann Neurol. 1986; 19:425–439.
Article7. Said G. Diabetic neuropathy--a review. Nat Clin Pract Neurol. 2007; 3:331–340.
Article8. Karsidag S, Morali S, Sargin M, Salman S, Karsidag K, Us O. The electrophysiological findings of subclinical neuropathy in patients with recently diagnosed type 1 diabetes mellitus. Diabetes Res Clin Pract. 2005; 67:211–219.
Article9. Abu-Shakra SR, Cornblath DR, Avila OL, Chaudhry V, Freimer M, Glass JD, et al. Conduction block in diabetic neuropathy. Muscle Nerve. 1991; 14:858–862.
Article10. Viader A, Sasaki Y, Kim S, Strickland A, Workman CS, Yang K, et al. Aberrant Schwann cell lipid metabolism linked to mitochondrial deficits leads to axon degeneration and neuropathy. Neuron. 2013; 77:886–898.
Article11. Feldman EL, Stevens MJ, Thomas PK, Brown MB, Canal N, Greene DA. A practical two-step quantitative clinical and electrophysiological assessment for the diagnosis and staging of diabetic neuropathy. Diabetes Care. 1994; 17:1281–1289.
Article12. Im S, Kim SR, Park JH, Kim YS, Park GY. Assessment of the medial dorsal cutaneous, dorsal sural, and medial plantar nerves in impaired glucose tolerance and diabetic patients with normal sural and superficial peroneal nerve responses. Diabetes Care. 2012; 35:834–839.
Article13. Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 2003; 26:Suppl 1. S5–S20.14. England JD, Gronseth GS, Franklin G, Miller RG, Asbury AK, Carter GT, et al. Distal symmetric polyneuropathy: a definition for clinical research: report of the American Academy of Neurology, the American Association of Electrodiagnostic Medicine, and the American Academy of Physical Medicine and Rehabilitation. Neurology. 2005; 64:199–207.
Article15. Gelding SV, Robinson S, Lowe S, Niththyananthan R, Johnston DG. Validation of the low dose short insulin tolerance test for evaluation of insulin sensitivity. Clin Endocrinol (Oxf). 1994; 40:611–615.
Article16. Dyck PJ, Davies JL, Clark VM, Litchy WJ, Dyck PJ, Klein CJ, et al. Modeling chronic glycemic exposure variables as correlates and predictors of microvascular complications of diabetes. Diabetes Care. 2006; 29:2282–2288.
Article17. Albers JW, Herman WH, Pop-Busui R, Martin CL, Cleary P, Waberski B, et al. Subclinical neuropathy among Diabetes Control and Complications Trial participants without diagnosable neuropathy at trial completion: possible predictors of incident neuropathy? Diabetes Care. 2007; 30:2613–2618.
Article18. Turner N, Heilbronn LK. Is mitochondrial dysfunction a cause of insulin resistance? Trends Endocrinol Metab. 2008; 19:324–330.
Article19. Kim B, McLean LL, Philip SS, Feldman EL. Hyperinsulinemia induces insulin resistance in dorsal root ganglion neurons. Endocrinology. 2011; 152:3638–3647.
Article20. Höke A. Animal models of peripheral neuropathies. Neurotherapeutics. 2012; 9:262–269.
Article21. Vincent AM, Hayes JM, McLean LL, Vivekanandan-Giri A, Pennathur S, Feldman EL. Dyslipidemia-induced neuropathy in mice: the role of oxLDL/LOX-1. Diabetes. 2009; 58:2376–2385.22. Vincent AM, Hinder LM, Pop-Busui R, Feldman EL. Hyperlipidemia: a new therapeutic target for diabetic neuropathy. J Peripher Nerv Syst. 2009; 14:257–267.
Article23. Fruchart JC, Sacks F, Hermans MP, Assmann G, Brown WV, Ceska R, et al. The Residual Risk Reduction Initiative: a call to action to reduce residual vascular risk in patients with dyslipidemia. Am J Cardiol. 2008; 102:10 Suppl. 1K–34K.
Article24. Woo V, Shestakova MV, Ørskov C, Ceriello A. Targets and tactics: the relative importance of HbA, fasting and postprandial plasma glucose levels to glycaemic control in type 2 diabetes. Int J Clin Pract. 2008; 62:1935–1942.
Article25. Nathan DM, Buse JB, Davidson MB, Ferrannini E, Holman RR, Sherwin R, et al. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2009; 32:193–203.
Article26. Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M, et al. Management of hyperglycaemia in type 2 diabetes: a patient-centered approach. Position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia. 2012; 55:1577–1596.
Article27. American Diabetes Association. Standards of medical care in diabetes--2012. Diabetes Care. 2012; 35:Suppl 1. S11–S63.28. El-Salem K, Ammari F, Khader Y, Dhaimat O. Elevated glycosylated hemoglobin is associated with subclinical neuropathy in neurologically asymptomatic diabetic patients: a prospective study. J Clin Neurophysiol. 2009; 26:50–53.
Article29. Smith AG. Impaired glucose tolerance and metabolic syndrome in idiopathic neuropathy. J Peripher Nerv Syst. 2012; 17:Suppl 2. 15–21.
Article30. Aaberg ML, Burch DM, Hud ZR, Zacharias MP. Gender differences in the onset of diabetic neuropathy. J Diabetes Complications. 2008; 22:83–87.
Article31. Booya F, Bandarian F, Larijani B, Pajouhi M, Nooraei M, Lotfi J. Potential risk factors for diabetic neuropathy: a case control study. BMC Neurol. 2005; 5:24.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Using Motivational Interviewing to Overcome Psychological Insulin Resistance
- Psychological Insulin Resistance: Key Factors and Intervention
- A Case of Acromegaly Presenting with Diabetic Ketoacidosis
- Relationship between Cardiovascular Autonomic Neuropathy and Diabetic Retinopathy in Patients with Non-Insulin Dependent Diabetes Mellitus
- Treatment Strategies for Diabetic Neuropathy